
Pneumonitis and cellular immunodeficiency triggered by the CDK 4/6 inhibitor Abemaciclib
Introduction
Breast cancer is the second most common cancer amongst women in the United States following non-melanoma skin cancer. There were an estimated 276,480 new cases in 2020, accounting for 15.3% of all new cancer diagnoses. Breast cancer contributed to 42,170 deaths, which accounts for 7% of all cancer deaths. The lifetime risk for developing breast cancer in females is about 13%. Six percent of women are stage IV at diagnosis. Five-year survival rate for metastatic breast cancer is 28%. About 30% of breast cancer survivors will develop metastatic disease at some point (1).
Fortunately, due to extensive ongoing research in the field, there have been many new therapies in the field of metastatic breast cancer which has drastically improved median survival in these patients. Cyclin dependent kinase (CDK) 4/6 inhibitors are a novel therapy in metastatic hormone positive breast cancer, and these include palbociclib, ribociclib and abemaciclib. CDKs are important regulatory enzymes in cell cycle transitions and cell division (2). Tumor suppressor retinoblastoma (Rb) protein controls key transition from growth-1 phase (G1) to the DNA synthesis, or S phase. During G1, a variety of growth signals can result in cyclin-D binding either CDK4 or CDK6 thereby causing phosphorylation of Rb and ultimately the release of E2F (transcription factor genes) and cell cycle progression. Selective inhibition of CDK4/6 causes the cell cycle to arrest in the G1 phase, resulting in reduced cell viability and tumor response (2).
Currently, the mainstay treatment for patients with metastatic hormone positive, human epidermal growth factor receptor 2 (HER-2) negative breast carcinoma includes one of the three CDK inhibitors approved by the Food and Drug Administration (FDA), palbociclib, ribociclib, or abemaciclib in combination with endocrine therapy. Hematologic toxicities primarily neutropenia, are common side effects of CDK4/6 inhibitors though usually mild to moderate and managed with dose interruption and/or dose reduction. A rare but serious adverse effect is pneumonitis, a severe inflammation of the lungs. Febrile neutropenia occurs less frequently than neutropenia alone if there is close monitoring of complete blood counts. Pneumonitis is even less common (3). Abemaciclib, the only CDK inhibitor approved for monotherapy, demonstrates greater selectivity for CDK than the other CDK inhibitors. This results in lower rates of hematologic toxicities, and therefore can be dosed continuously, as ribociclib and palbociclib require treatment breaks (3). In this case, we present a patient who developed hypogammaglobinemia and severe pneumonitis despite dose interruption of abemaciclib.
Case presentation
A 65-year-old female with history of rheumatoid arthritis on hydroxychloroquine and steroids, gastroesophageal reflux disease, hypertension, and metastatic breast cancer presented to the emergency room with fever. She had a history of right-sided estrogen receptors (ER) positive (strong intensity in 98.3% of cells)/progesterone receptors (PR) positive (strong intensity in 86.2% of cells), HER-2/neu negative invasive lobular carcinoma diagnosed 5 years prior. She was initially staged as IIB (T3, N0, M0). She had a pre-operative computed tomography (CT) chest with IV contrast that showed subpleural irregular opacities with areas of honeycombing consistent with underlying mild pulmonary fibrosis. She first underwent right partial mastectomy with axillary sentinel node biopsy which revealed an 8.5-centimeter (cm) tumor with positive margins and 5 out of 6 lymph nodes positive. She was therefore upstaged to IIIA, pT3N2M0. The patient then underwent a right modified radical mastectomy with axillary lymph node dissection. Pathology revealed invasive lobular carcinoma staining ER positive, PR positive, and HER-2/neu negative by immunohistochemistry (IHC). Margins were positive and 16 out of 23 lymph nodes were involved, further upstaging to IIIC (pT3, N3, M0).
After radical mastectomy, she had adjuvant chemotherapy with 21-day cycle for four cycles of dose dense doxorubicin [60 milligram (mg) per meter squared] and cyclophosphamide (600 mg/m2) with Neulasta for marrow convalescence followed by 14-day cycle for four cycles of dose dense paclitaxel (175 mg per meter squared). After completion, she received radiotherapy to the chest wall [5,000 centigray (cGy) with 25 fractions of 100 cGy per day over a course of 48 days], supraclavicular fossa (5,000 cGy with 25 fractions of 200 cGy per day over a course of 48 days) and a boost to the mastectomy scar (1,000 cGy with 5 fractions of 200 cGy per day over a course of 4 days). Afterwards, the patient was prescribed adjuvant anastrozole 1 mg, an aromatase inhibitor, which was taken daily for 5 years along with calcium and vitamin D. She was also started on Denosumab as her dual energy X-ray absorptiometry (DEXA) scan revealed osteopenia.
Five years from initial diagnosis, the patient presented to the emergency department (ED) complaining of worsening lower back pain and was found to have numerous osseous metastases on magnetic resonance imaging (MRI) of the lumbar spine. There was no evidence of cord compression. MRI of the cervical and thoracic spine was done which was also positive for metastatic disease. CT-guided biopsy of an L2 lesion confirmed metastatic invasive lobular carcinoma of the breast consistent with a breast primary, ER positive, PR negative and HER-2 not overexpressed. Subsequently, she underwent stereotactic body radiation therapy (SBRT) 3,000 cGy in 10 fractions over 14 elapsed days of the L1-S2 lesions for palliation. CT chest showed stable post-treatment subpleural fibrotic changes in right lung and mild dependent atelectasis and/or subpleural fibrotic change at the left base similar to prior. No inflammatory consolidation, suspicious lung nodule or pleural effusion seen. CT abdomen and pelvis did not reveal any evidence of metastatic disease. After completion of SBRT, the patient was started on fulvestrant (500 mg intramuscularly) and denosumab (Xgeva 120 mg subcutaneously every 4 weeks) with plans to initiate abemaciclib 150 mg twice a day. Abemaciclib was delayed 2 months due to heightened coronavirus disease 2019 (COVID-19).
Approximately 1 month after initiating abemaciclib, patient presented to the ED for shortness of breath and fever of 102 Fahrenheit for five days. Two weeks prior she was admitted at another hospital where she was treated for pneumonia with intravenous IV vancomycin and ceftriaxone and placement of a bronchial stent. Her symptoms worsened despite completing a course of cefdinir. At the time of admission, the complete blood count revealed white blood cell count (WBC) 5.0 thousand/cubic millimeter (k/mm3) [normal range (NR) 4.5–10.0 k/mm3], hemoglobin (Hgb) 10.1 grams/deciliter (g/dL) (NR 12.0–16.0 g/dL), platelet count 105 g/dL (NR 150–440 g/dL), absolute neutrophil count (ANC) 2.3 k/mm3 (NR 2.0–8.0 k/mm3), absolute lymphocyte count (ALC) 1.9 k/mm3 (NR 1.0–4.8 k/mm3), blood urea nitrogen (BUN) 11 mg/dL (NR 7.0–18.0 mg/dL) and creatinine 1.22 mg/dL (NR 0.6–1.1 mg/dL). A chest X-ray revealed patchy upper lobe and peripheral predominant airspace disease. She was started on IV vancomycin and piperacillin/tazobactam. Subsequent CT of the chest revealed significant infiltration in both upper lobes having the appearance of pneumonia. Emphysematous changes and interstitial fibrotic changes seen in both lower lobes with an inflammatory or edematous superimposed process.
After further questioning, the patient had recently discontinued her abemaciclib due to ongoing worsening symptoms after 2 weeks, including hypoxic respiratory failure requiring supplemental oxygen via nasal cannula.
The patient was started on corticosteroids and bronchodilators. Blood cultures were negative at 5 days, viral respiratory PCR was negative for viral pathogens and sputum cultures, all obtained on admission, were contaminated with oropharyngeal flora. At that time, ANC was 1.28 k/mm3. Serum immunoglobulin levels were measured to further investigate the etiology of patient’s worsening respiratory status. Total immunoglobulin G (IgG) measured 163 mg/dL (NR 553–1,360 mg/dL) IgA level was 53 mg/dL (NR 71–374 mg/dL), and all subtypes of IgG were decreased. Human immunodeficiency virus (HIV) was negative. Patient was treated with one dose of 90 g of intravenous immunoglobulin (IVIG) and was also started empirically on trimethoprim-sulfamethoxazole due to concern for possible Pneumocystis jiroveci pneumonia (PJP). Both hypogammaglobinemia and severe lymphopenia were attributed to possible immunosuppression due to chronic steroid use, or a sequela of CDK inhibitors causing severe cellular immunodeficiency. The patient’s respiratory status worsened requiring five liters of oxygen via nasal cannula and a chest X-ray showed low lung volumes and bilateral chronic appearing opacities. Bronchoscopy with bronchoalveolar lavage and cultures was recommended by the pulmonary team, but patient declined to undergo the procedure. Subsequently, the patient’s hospital course was complicated by atrial fibrillation with rapid ventricular response and decline in her respiratory status, requiring high flow oxygen via nasal cannula, followed by bilevel positive airway pressure (BIPAP). Additional pertinent laboratory testing during this time revealed fungitell level 250 pg/mL, ANC 19.5 k/mm3 and ALC 0.6 k/mm3. The patient decided to pursue comfort measures and died shortly thereafter.
Discussion
We report a fatal case of severe lung injury with presumed superimposed fungal respiratory infection in a 65-year-old female with metastatic ER positive/HER-2 negative breast cancer who received abemaciclib 150 mg twice a day for 10 days. The patient did have a history of asymptomatic underlying mild pulmonary fibrosis due to rheumatoid arthritis (Figures 1A,1B), but after administration of the CDK4/6 inhibitor, the patient’s respiratory status deteriorated significantly, and lung imaging revealed extensive interstitial pulmonary scarring (Figure 1C,1D).
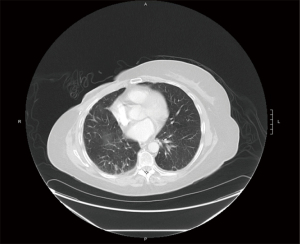
There is much data linking anti-neoplastic medications to pulmonary toxicity. Commonly used anti-cancer drugs associated with drug induced lung injury include gemcitabine, taxotere, bleomycin and immune check point inhibitors. There is a scarcity of data regarding prevalence of pneumonitis or severe lung injury linked to CDK4/6 inhibitors, likely due to their recent usage. In the MONARCH 3 trial with abemaciclib in metastatic breast cancer, there was one death reported which was attributed to pneumonitis (4). In a case report describing the use of abemaciclib in metastatic breast cancer, Ahsan et al. reported development of respiratory distress and ground glass opacities in a 52-year-old woman with non-revealing infectious work-up and no prior pulmonary disease, which was ultimately fatal (5). Another case presented by Gong et al. reported development of pneumonitis after eight cycles of palbociclib in combination with fulvestrant which resolved with conservative management and discontinuation of the CDK inhibitor (6). Our patient had evidence of mild pulmonary fibrosis at baseline, which may have predisposed her to develop chemically induced pneumonitis. Presumably, that patient likely developed pneumonia secondary to neutropenia, lymphopenia and hypogammaglobulinemia which may have been multi-factorial related to CDK4/6 inhibitor as well as chronic immunosuppression due to treatment of her underlying rheumatoid arthritis.
Throughout the literature, the most common hematologic side effect of CDK inhibitors was neutropenia, which can be explained by the integral role of CDK6 in proliferation of hematologic precursors. CDK4/6 inhibitors lead to the cytostatic effect on neutrophil precursors resulting in pharmacologic quiescence. Most cases of CDK inhibitor induced neutropenia is mild and has an uncomplicated course. It is usually managed with dose interruption and/or dose reduction to prevent future neutropenic episodes (3). Throughout our patient’s hospitalization, the total ANC fluctuated, with the lowest at 1,028 cells/microliter (normal >1,500 cells/microliter), but at the time of patient’s death, ANC was elevated at 19,000 cells/microliter, which did not correlate with the most likely opportunistic superinfection. Beta-D-glucan was elevated at 250 pg/mL, which may suggest underlying fungal infections such as candidiasis and aspergillosis, but the specificity of this assay is limited with a high false positive rate (7).
With deterioration in her clinical status, further investigation revealed hypogammaglobulinemia, in addition to her known neutropenia and lymphopenia. Circulatory IgG and IgM antibodies are usually found in response to fungal antigens in individuals with symptomatic systemic fungal infections such as aspergillosis, blastomycosis, candidiasis, cryptococcosis and histoplasmosis (8). The patient’s inability to mount an effective immune response to a presumably latent fungal infection is due to several causes including the essential immunodeficiency characterized by hypogammaglobulinemia and lymphopenia.
Hypogammaglobulinemia has mostly been associated with B-cell depleting antineoplastic therapy such as rituximab in the literature. Rituximab is a monoclonal antibody directed against CD20 antigen on B cells, and is FDA approved for treatment of non-Hodgkins lymphoma, chemo-resistant follicular lymphoma, B-cell lymphoma, multiple sclerosis, and rheumatoid arthritis. A literature review was published by Sacco and Abraham examining the patient treated with rituximab who were high risk for developing symptomatic hypogammaglobulinemia, which was defined as patient with low immunoglobulins who developed typical infections associated with immunoglobulin deficiency (9). Most of the studies in the review defined hypogammaglobulinemia as a serum IgG less than 5.65–7 g/dL. A major risk factor found in the literature review included low baseline low levels of IgG and IgM, with persistent hypogammaglobulinemia defined as hypogammaglobulinemia lasting more than 6 months after treatment with rituximab; these patients also developed the more severe bronchopulmonary infections (9). There is little to no data showing any association of hypogammaglobulinemia associated with cyclin dependent kinase inhibitors, while neutropenia and severe interstitial pneumonitis was recorded initial clinical trials.
Neutropenia occurred in 41% of patients receiving abemaciclib plus an aromatase inhibitor in MONARCH 3, 46% of patients receiving abemaciclib plus fulvestrant in MONARCH 2, and 37% of patients receiving single agent abemaciclib in MONARCH 1. Febrile neutropenia was reported in less than 1% of patients receiving abemaciclib in all MONARCH studies (3,4,10).
Severe, life threatening or fatal interstitial lung disease (ILD) and/or pneumonitis can occur in patients receiving abemaciclib and other CDK4/6 inhibitors. In MONARCH 1, MONARCH 2 and MONARCH 3, 3.3% of patients developed ILD of any grade, 0.6% had grade 3 or 4, and 0.4% were fatal. Dose interruption or reduction is recommended for patients who develop recurrent or persistent grade 2 ILD and permanent discontinuation for grade 3 or 4 (3,4,10).
In MONARCH 2, 43% of patients receiving abemaciclib and fulvestrant developed infections of all grades, 5% had grade 3 and less than 1% grade 4. Placebo plus fulvestrant arm had 25% of all grades, 3% grade 3 and less than 1% grade 4. Lymphopenia of all grades was found in 63% of patients given abemaciclib and fulvestrant, 12% had grade 3 and less than 1% grade 4. Whereas placebo and fulvestrant arm had 32% lymphopenia of all grades, 2% grade 3 and 0% grade 4 (9).
Conclusions
Although CDK inhibitor induced lung injury is documented as a rare side effect in PALOMA and MONARCH 3, there is very little data about how these medications effect those with underlying pulmonary fibrosis. One should strongly consider screening patients for baseline pulmonary scarring before starting any CDK inhibitors, as the drug-induced pneumonitis may be life threatening, and as seen in our patient, rapidly deteriorating. It was also observed in clinical trials that palbociclib can induce severe lymphopenia and severe immunodeficiency characterized by decreased lymphocyte count, more specifically decreased CD4 T lymphocytes. It should be acknowledged that abemaciclib can not only have similar effects, but can also cause life threatening hypogammaglobulinemia, leading to opportunistic fungal infections. Consider monitoring lymphocyte count and immunoglobulin levels in patients receiving abemaciclib. There may be a role for antifungal prophylaxis in conjunction with Pneumocystis pneumonia (PCP) prophylaxis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-21-19/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-21-19/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was waived as the patient was a widow who passed away more than a year ago. The patient’s consent was not obtained as the author cannot reach her family by the numbers listed on patient’s chart.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- SEER Cancer Stat Facts: Female Breast Cancer. National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/statfacts/html/breast.html
- Spring LM, Wander SA, Zangardi M, et al. CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr Oncol Rep 2019;21:25. [Crossref] [PubMed]
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res 2017;23:5218-24. [Crossref] [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Ahsan I, Malik F, Jafri S. Palbociclib related pneumotoxicity: a rare side effect. Am J Respir Crit Care Med 2017;195:A45546.
- Gong J, Cho M, Yu KW, et al. A single institution experience with palbociclib toxicity requiring dose modifications. Breast Cancer Res Treat 2018;168:381-7. [Crossref] [PubMed]
- Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1-->3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis 2005;41:654-9. [Crossref] [PubMed]
- Lanternier F, Cypowyj S, Picard C, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr 2013;25:736-47. [Crossref] [PubMed]
- Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy 2018;10:713-28. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
Cite this article as: Ashraf S, Biglow L, Dotson J, Tirona MT. Pneumonitis and cellular immunodeficiency triggered by the CDK 4/6 inhibitor Abemaciclib. Transl Breast Cancer Res 2022;3:9.


