
Round table discussion: strategies for the treatment of HER2-positive advanced breast cancer in the rising age of antibody-drug conjugates
Introduction
Based on the mechanism of action, anti-HER2-targeted agents can be divided into macromolecular antibodies, small molecule tyrosine kinase inhibitors (TKIs) and antibody-drug conjugates (ADCs). Ten years ago, when trastuzumab was the mainstream treatment, the median survival of patients with HER2-positive advanced breast cancer was only about 3 years (1,2). With the extensive application of trastuzumab combined with pertuzumab in first-line therapy in clinical practice, the options of second-line and subsequent anti-HER2-targeted therapies such as TKIs and T-DM1 are increasing. Real-world studies have shown that the median survival of patients with HER2-positive advanced breast cancer has been extended to about 5 years (3-5). A new generation of HER2-targeted ADC drugs, such as trastuzumab deruxtecan (T-DXd, DS-8201) and disitamab vedotin (RC-48), are expected to improve the prognosis of patients with HER2-positive advanced breast cancer by overcoming the heterogeneity of HER2-positive tumors with bystander effect due to the innovation of coupling technology.
The published results of DESTINY-Breast03 (6) shows, compared with the traditional anti-HER2 ADC drug T-DM1, T-DXd has significant advantages in the extension of PFS and the improvement of overall survival (OS), providing a better treatment option. These advantages are related to the different structures and properties between T-DXd and T-DM1. T-DXd is a novel antibody-drug conjugate, which delivers a topoisomerase I inhibitor with a high drug-to-antibody ratio of 7–8 with a tetrapeptide linker. The potency of the active payload, as well as its significant bystander effect, improved the anti-tumor activity significantly.
The results of DESTINY-Breast03 were only presented on the European Society for Medical Oncology (ESMO) CONGRESS 2021 and San Antonio Breast Cancer Symposium (SABCS) 2021, the full text has not been published. In addition, with the widespread adoption of (neo) adjuvant therapy trastuzumab + pertuzumab for HER2-positive early-stage breast cancer, as well as postoperative intensive treatments with ADC drugs (such as T-DM1) and TKI drugs (such as neratinib), the status and application strategies of different types of anti-HER2-targeted therapy in HER2-positive advanced breast cancer need to be re-examined and reconsidered.
This round table discussion was held to analyze the DESTINY-Breast03 clinical study deeply and compare it with past HER2-positive advanced breast cancer clinical trials in terms of study design, characteristics of patients, primary results, safety, etc. Five demanding questions about the strategies for the treatment of HER2-positive advanced breast cancer in the rising age of ADCs were provided and discussed. Experts took out their ideas about how to balance the efficacy, safety, and feasibility to give patients the greatest benefit and prolonged survival with better quality of life.
DESTINY-Breast03 clinical study analysis
The DESTINY-Breast03 study (6,7) is a head-to-head comparison of the efficacy and safety of two ADC drugs, T-DXd and T-DM1, in breast cancer in patients previously treated with trastuzumab.
The study enrolled a total of 524 patients with HER2-positive advanced breast cancer who had been previously treated with trastuzumab and taxanes and more than 60% of patients in both groups in the DESTINY-Breast03 study received pertuzumab, representing the population not previously covered by relevant studies of pyrotinib [PHOEBE study (8) and PHENIX study (9)] and T-DM1 [EMILIA study (10,11)]. Therefore, the results of the DESTINY-Breast03 study can better reflect the current clinical practice that after the standard first-line treatment of trastuzumab + pertuzumab, T-DXd is significantly superior to T-DM1 as the second-line treatment. In addition, about 22% patients with brain metastases who were stable after local treatment were included in this study, which also provides reference for the treatment of ADC drugs in patients with HER2-positive brain metastases. Eligible patients were randomly divided into T-DXd group (5.4 mg/kg Q3w) and T-DM1 group (3.6 mg/kg Q3w) at a 1:1 ratio.
The primary endpoint of the study was progression-free survival (PFS) by blinded independent central review (BICR). When 335 (100%) PFS event finally occurs in the statistical analysis plan, P<0.049998 or HR <0.807, which could be considered as a significant statistically difference; therefore, a very strict P value (P<0.000204 or HR <0.615) was assigned for the interim analysis (70% PFS events occurred); it is such an excitement to find that the PFS data has reached statistical difference in the first interim analysis with HR =0.28, P=7.8×10−22, which could be concluded as the final PFS analysis. With the median follow-up of 16.2 months in T-DXd group, the BICR-assessed data showed significant clinical improvement in PFS in the T-DXd group compared with T-DM1. The median PFS of the T-DXd group assessed by the investigators was 25.1 months, which is the longest PFS value reported in any clinical study of HER2-positive advanced breast cancer in the second and posterior line. The BICR-assessed median PFS in the T-DM1 control group was 6.8 months, which was significantly lower than the 9.6 months reported in the EMILIA study (10). The main reasons included that patients previously treated with pertuzumab and failed TKIs treatment were not included in the EMILIA study, and most of the patients (61%) received first-line or second-line treatment in the stage of relapse and metastasis; however, in the DESTINY-Breast03 study, >60% of patients were previously treated with pertuzumab, 13.7% were previously treated with TKIs, and only 47.9% received first-line or second-line treatment, while more than half patients received more than 2 lines of treatment. The study was designed with three stratification factors, including hormone receptor status (ER+ about 50%), whether patients have been previously treated with pertuzumab (about 60% of patients have been treated) and whether there were visceral metastases (about 70% of patients experienced visceral metastases). Subgroup analysis showed that in each subgroup, T-DXd could bring consistent significant PFS benefit.
The DESTINY-Breast03 study allowed patients with clinically stable and locally treated brain metastases to be enrolled. Forty-three cases (16.5%) in the T-DXd group and 39 cases (14.8%) in the T-DM1 group were reported to had baseline brain metastases at enrollment. The median PFS of the two groups were 15.0 and 3.0 months respectively (HR =0.25, 95% CI: 0.13–0.45). There were 36 patients in both groups with evaluable intracranial lesions, of which 10 patients (27.8%) in the T-DXd group achieved complete remission (CR) of brain metastases; the number of patients whose brain metastases were selected as target lesions at baseline were 21 and 23 cases in the two groups, respectively, of which 17 cases (81.0%) in the T-DXd group decreased the volume of the target intracranial lesions by more than 30%. Results showed that T-DXd has higher therapeutic activity for patients with HER2-positive brain metastases. It is worth noting that all the patients with brain metastases included in the DESTINY-Breast03 study were clinically stable and locally treated, yet patients with active brain metastases were not included, including those with untreated brain metastases and those with further local progression after local treatment. For patients with active brain metastases, a phase II clinical study (HER2CLIMB-04, NCT04539938) of small molecule tucatinib combined with ADC drug T-DXd is currently underway to explore a better treatment strategy of HER2-positive breast cancer with brain metastases.
The T-DXd group showed a tendency to improve OS, but the analysis was not mature and had no statistical significance. T-DXd is currently available in the United States, Japan and Europe, and patients in the T-DM1 control group were likely to choose T-DXd as the later-line treatment. DESTINY-Breast01 (12) showed that in HER2-positive breast cancers that failed multiple lines of treatment, T-DXd could still reach a median PFS of 16.4 months. Therefore, in the DESTINY-Breast03 study, whether T-DXd can greatly improve survival requires further follow-up and data update.
The ORR (CR + PR) of the T-DXd group was 79.7%, which was significantly higher than 34.2% of the T-DM1 group, suggesting that the T-DXd group realized a stronger tumor reduction effect in the second-line and subsequent treatments. In the visceral metastasis subgroup, the ORRs of the two groups were 77.4% and 29.1% respectively. The reduction of tumor burden is conducive to disease control and improvement of tumor-related quality of life.
The safety profile of the most common adverse events of T-DXd was consistent with the results from previous clinical trials, and no new safety events were identified. The most common grade 3 or higher treatment-emergent adverse events in the T-DXd group were neutropenia (19.1%), thrombocytopenia (7.0%), leukopenia (6.6%), and nausea (6.6%). The most common adverse events with treatment discontinuation for T-DXd was interstitial lung disease (ILD)/pneumonitis (8.2%) and for T-DM1 was thrombocytopenia (2.7%). The most common adverse events with dose reduction for T-DXd were nausea (6.2%) and neutropenia (3.5%) and for T-DM1 were thrombocytopenia (4.2%) and alanine transaminase (ALT) and aspartate transaminase (AST) increased (2.7% each). It is suggested that the toxicity and side effects are closely related to the chemotherapeutic drugs coupled with the ADCs. Grade 4–5 ILD did not occur, and the ILD characteristics were significantly improved compared to those of patients treated with multiple lines in DESTINY-Breast01 (12). In addition, the quality of life jeopardized by toxicity side effects during long-term treatment should be paid attention to, and the occurrence of ILD, an adverse event of special concern, should not be ignored.
Despite the breakthrough of T-DXd, all patients will eventually progress on current therapeutic options. We look forward to new strategies to overcome HER2 therapy resistance and tumor heterogeneity. The innovation drugs with brand new mechanisms, combinations of anti-HER2 therapy with immune checkpoint inhibitors, CDK4/6 inhibitors, and PI3K/AKT/mTOR inhibitors are promoted for clinical trials.
In conclusion, in the DESTINY-Breast03 study, T-DXd has set a new PFS record for HER2-positive advanced breast cancer and will establish its international status as a second-line standard treatment. According to the results of this study, T-DXd has replaced T-DM1 as the best choice for second-line therapy. However, due to drug accessibility, economic burden and other factors, there are still some difficulties in widely adoption of this drug in second-line treatment in China. In addition, more diverse clinical studies of T-DXd are actively conducting in the field of HER2-positive advanced breast cancer. For example, DESTINY-Breast02 focuses on the efficacy of T-DXd in patients who have failed T-DM1 therapy (13). DESTINY-Breast09 further explores the potential of T-DXd in first-line therapy (13). In the future, patients are encouraged to actively participate in the clinical research of ADCs.
Round table discussion of five demanding questions
(I) How to evaluate the value of PFS, OS and ORR as efficacy indicators and their impact on clinical practice in the context of the DESTINY-Breast03 study of HER2-positive advanced breast cancer?
Expert opinion
Most experts regard PFS as the primary endpoint of clinical studies. For HER2-positive advanced breast cancer, OS is relatively long and may be affected by multi-line therapies in the disease progress. Therefore, PFS can more objectively reflect the efficacy of drugs with a shorter observation period, which is conducive to saving samples and speeding up drug approval. In the DESTINY-Breast03 study (6), HR =0.28 in two groups and the difference was statistically significant. The median PFS of the T-DXd group assessed by the investigator was 25.1 months, which is the longest PFS reported in the current clinical studies of second-line and post-line treatment of HER2-positive advanced breast cancer, indicating that the T-DXd group has a significant improvement in clinical efficacy compared with T-DM1 group. The T-DXd group showed a tendency to improve OS, but the analysis was not mature and there was no statistical difference. In addition, OS may be influenced by the baseline characteristics of patients enrolled, including the number of previous treatment lines and differences in previous anti-HER2 therapies, and other factors such as some patients in the control group would choose T-DXd as the follow-up treatment after they left the group. There may not be a significant benefit of OS in the final data of DESTINY-Breast03 study. Therefore, experts believe that the PFS data in this study are sufficient to show the efficacy of T-DXd, which can be used for drug approval so as to treat and benefit patients earlier.
Some experts hold a different point of view, believing that OS is generally recognized as the best evaluation indicator of efficacy. It is suggested that if the therapeutic advantage of T-DXd and T-DM1 is large enough, the obvious improvement of OS shall be observed. In the previous study of trastuzumab (H0648g) (1) and the study of trastuzumab + pertuzumab (CLEOPATRA) (3,4), some enrolled patients also crossed over to the trial group, both leading to a significant improvement in OS, and the magnitude of OS benefit is greater than that of PFS. Whether T-DXd can greatly improve survival requires further follow-up and data update. Experts noted that OS is the “gold standard” for treating advanced breast cancer, but it can be affected by many factors. In RCT studies, early death events usually occurred among patients with large tumor burden, rapid disease progression and previous multi-line treatment. Whether there is a significant difference in OS between the trial group and the control group is also closely related to the treatment efficacy of the control group. For example, in the T-DM1 study (EMILIA) (10,11), almost all patients in the lapatinib + capecitabine group had progression and death events during the follow-up, so the OS curve dropped rapidly. In the T-DM1 group, a small number of patients who responded to treatment and were still alive would indicate a significant improvement in OS.
Most experts agree that, although objective response rate (ORR) is not usually used as a primary endpoint in clinical studies, it is more important in clinical practice. Especially for patients with large tumor burden and rapid disease progression. The ORR (CR + PR) of T-DXd group was 79.7%, significantly higher than 34.2% of T-DM1 group, suggesting that T-DXd group can effectively reduce the tumor burden, relieve the tumor-related symptoms, improve the quality of life in the second-line treatment so as to meet the need of advanced breast cancer treatment. Moreover, achieving tumor reduction and control as early as possible can give patients and doctors more confidence in continuing treatment.
(II) In patients with resistance to both antibodies and TKIs, can ADCs be used as the first choice of treatment?
Expert opinion
All experts agreed that ADCs shall be the preferred treatment for patients with resistance to both antibodies and TKIs. Currently, approved ADCs targeting HER2 include T-DM1 (10,11) and T-DXd (6,12). Both DESTINY-Breast01 (12) and DESTINY-Breast03 (6) have shown that T-DXd has a better efficacy for patients with these characteristics. Although patients who failed TKIs treatment were not included in the T-DM1 study (EMILIA) (10,11), T-DM1 can also be regarded as a treatment option from the perspective of drug mechanism. Re-challenge of macromolecular antibodies and TKIs may also be a treatment option in patients who have no access to ADCs. Patients who used to achieve pathology complete response (pCR) or near pCR in the neoadjuvant treatment may get the benefit from the re-challenge of trastuzumab + pertuzumab.
Although T-DXd is not available at present, patients are encouraged to actively participate in the clinical research of ADCs, such as disitamab vedotin (RC-48) (14), SYD985 (15), ARX788 (16), A166 (17) and MRG002 (18) other related clinical studies on the treatment of HER2-positive breast cancer, which can provide patients with the opportunity to benefit from treatment and also promote the development of innovative drugs in China (Table 1).
Table 1
| Variables | T-DXd (6,12) | T-DM1 (10,11) | RC48 (14) | SYD985 (15) | ARX788 (16) | A166 (17) | MRG002 (18) |
|---|---|---|---|---|---|---|---|
| Antibody | Trastuzumab | Trastuzumab | Disitamab | Trastuzumab | Trastuzumab | Trastuzumab | Trastuzumab |
| Linker | Cleavable | Non-cleavable | Cleavable | Cleavable | Non-cleavable | Cleavable | Cleavable |
| DAR | 8 | 3.4 | 4 | 2.4–2.8 | 1.9 | 2 | ~3.8 |
| Payload | Highly active topoisomerase I inhibitor deruxtecan | Microtubule inhibitor DM1 | Microtubule inhibitor MMAE | Alkylating agent duocarmycins | Microtubule inhibitor amberstatin | Microtubule inhibitor MMAF | Microtubule inhibitor MMAE |
| Bystander effect | Yes | No | Yes | Yes | No | Yes | Yes |
| Clinical development status | Global launched Phase III trial ongoing in China | Launched | Phase II/III trial ongoing in China | Global Phase III trial completed (TULIP trial) | Phase II/III trial ongoing in China | Phase II trial ongoing in China | Phase II trial ongoing in China |
ADCs, antibody-drug conjugates; T-DXd, trastuzumab deruxtecan; T-DM1, trastuzumab emtansine; RC48, disitamab vedotin; SYD985, (Vic-)trastuzumab duocarmazine; DAR, drug-to-antibody ratio; MMAE, monomethyl auristatin E; MMAF, monomethyl auristatin F.
(III) After the development of antibody therapy, which therapy should be the preferred second line treatment, TKIs or ADCs? (Figure 1)
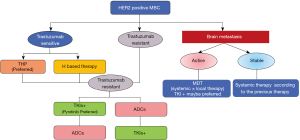
Expert opinion
The T-DM1 study (EMILIA) (10,11) and the pyrotinib + capecitabine study (PHOEBE) (8) only enrolled patients who were previously treated with trastuzumab. For trastuzumab-resistant patients, current clinical studies show that T-DM1 is superior to lapatinib + capecitabine (EMILIA study), pyrotinib + capecitabine is superior to lapatinib + capecitabine (PHOEBE study), and T-DXd is superior to T-DM1 (DESTINY-Breast03 study). However, there is no direct head-to-head comparison of pyrotinib + capecitabine with T-DXd or T-DM1 yet. Currently, the reported median PFS of T-DXd group was 25.1 months (DESTINY-Breast03 study), higher than 12.5 months (PHOEBE) of pyrotinib + capecitabine group. More than 60% of patients in both groups in the DESTINY-Breast03 study received pertuzumab, representing the population not previously covered by relevant studies of pyrotinib [PHOEBE study (8) and PHENIX study (9)] and T-DM1 [EMILIA study (10,11)]. The results of the DESTINY-Breast03 study can better reflect the current clinical practice that after the standard first-line treatment of trastuzumab + pertuzumab, T-DXd is significantly superior to T-DM1 as the second-line treatment. Therefore, some experts recommend T-DXd as the first choice for second-line treatment after disease progression following antibody therapy.
Some experts hold a different opinion and think TKIs should be the first choice. Pyrotinib is currently the most widely used second-line treatment for patients who have been treated with trastuzumab, especially those with trastuzumab resistance. Two phase III RCT studies, PHOEBE study (8) and PHENIX study (9), confirmed that pyrotinib + capecitabine had a definite effect on patients who have been treated with trastuzumab, and both PFS and OS were significantly improved. In HER2-positive brain metastases, especially patients with active brain metastases, small molecule TKIs has an advantage. All the patients included in the DESTINY-Breast03 study were clinically stable and locally treated patients with brain metastases. For patients with active brain metastases, a phase II clinical study (HER2CLIMB-04, NCT04539938) of small molecule tucatinib combined with ADC drug T-DXd is currently underway. Furthermore, DESTINY-Breast01 study (12) showed that T-DXd still had a median PFS of 16.4 months among HER2-positive breast cancers that had failed trastuzumab, T-DM1 and TKIs multiline therapies, indicating a promising efficacy. However, there is no evidence from clinical trial of TKIs treatment after ADC treatment fails. While combining evidence-based medicine and drug accessibility, TKIs would be a preferred option after disease progression following antibody therapy.
All experts support that the choice between TKIs and ADCs in clinical practice should be based on specific circumstances, such as drug availability, economic status, and control of brain metastases.
(IV) What is the prospect of ADC drug T-DXd in first-line therapy?
Expert opinion
Based on current guidelines and clinical data, trastuzumab + pertuzumab macromolecular antibody therapy is recommended for patients who are sensitive to trastuzumab therapy and are eligible for trastuzumab reuse. However, it is worth exploring whether dual-target therapy after relapse and metastasis can achieve the PFS and OS benefits reported in the CLEOPATRA study in patients previously treated with trastuzumab in the early stages. The study of pyrotinib + capecitabine (PHOEBE) also included some patients who had been treated with trastuzumab but were still eligible for reuse without the definite resistance of trastuzumab. And the ongoing T-DXd study (DESTINY-Breast09) is planned to further explore efficacy and benefits of T-DXd in the first-line treatment.
Most experts believe that first-line treatment for HER2-positive advanced breast cancer takes a long period of time, so the decision of first-line treatment shall be made not only based on efficacy, but also the balance of safety and quality of life. Current data and clinical experience suggest that long-term treatment with dual antibodies results in the best tolerability and quality of life for patients. T-DXd, as an ADC agent, has some characteristics of side effects of chemotherapy drugs, such as fatigue and loss of appetite during long-term use. These negative effects on quality of life may hinder the long-term use of T-DXd in first-line treatment. Some experts think that as long as T-DXd can bring significant efficacy benefits with good tolerability, it shall be a preferred option in first-line treatment.
(V) The current treatment options are so diversified and complex, what are the treatment options of HER2-positive early-stage breast cancer after relapse and metastasis (Figure 2)
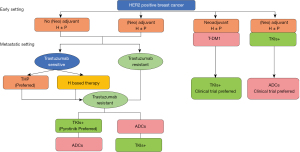
Expert opinion
After neoadjuvant therapy with trastuzumab + pertuzumab for HER2-positive early-stage breast cancer, the current standard of treatment for non-PCR patients is T-DM1 postoperative intensive therapy. Some patients may receive adjuvant intensive treatment with neratinib in the adjuvant treatment stage. There is no clinical data on how to rescue these patients after relapse and metastasis. With a certain degree of resistance to antibody, T-DM1 and/or TKIs, these patients may require individualized treatment based on the actual situation. Patients who had not been completely relieved in the neoadjuvant therapy with trastuzumab + pertuzumab or progressed during the adjuvant setting with antibodies, T-DM1 or neratinib, tend to be with poor prognosis due to the resistance to the multiple anti-HER2 targets.
The DESTINY-Breast01 clinical study (12) suggests that such patients may benefit from T-DXd treatment. Experts think that it is also a treatment option to encourage patients to participate actively in relevant clinical studies.
After all, DESTINY-Breast03 study shows T-DXd has significant advantages in the improvement of PFS and OS. It means some strategies for the treatment of HER2-positive advanced breast cancer should be changed in the rising age of ADCs. All experts agreed that ADCs shall be the preferred treatment for patients with resistance to both antibodies and TKIs. Although T-DXd is not available at present in China, patients are encouraged to actively participate in the clinical research of ADCs. But there are some controversial issues about the prospect of ADC drug T-DXd in first-line therapy and the preferred second line treatment after the development of antibody therapy. To be honest, this roundtable discussion is only a few people’s viewpoints, not up to the category of consensus. The strategies for the treatment of HER2-positive advanced breast cancer in the rising age of ADCs need to be investigated further.
Experts participating in the discussion (sorted by surname)
Chunfang Hao (Tumor Hospital of Tianjin Medical University), Zefei Jiang (Fifth Medical Center of Chinese PLA General Hospital), Man Li (Second Affiliated Hospital of Dalian Medical University), Qiao Li (Cancer Hospital of Chinese Academy of Medical Sciences), Qiang Liu (Sun Yat-sen Memorial Hospital of Sun Yat-sen University), Tao Sun (Liaoning Cancer Hospital), Haibo Wang (The Affiliated Hospital of Qingdao University), Shu Wang (Peking University People’s Hospital Breast Center), Shusen Wang (Sun Yat-sen University Cancer Center), Tao Wang (Fifth Medical Center of PLA General Hospital), Xiaojia Wang (Cancer Hospital Affiliated to Chinese Academy of Sciences), Min Yan (The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital), Ying Yan (Peking University Cancer Hospital & Institute), Yongmei Yin (the First Affiliation Hospital with Nanjing Medical University), Peng Yuan (Cancer Hospital of Chinese Academy of Medical Sciences), Pin Zhang (Cancer Hospital of Chinese Academy of Medical Sciences).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (https://tbcr.amegroups.com/article/view/10.21037/tbcr-21-45/coif). JL serves as the unpaid managing editor of Translational Breast Cancer Research from November 2019 to October 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [Crossref] [PubMed]
- Bredin P, Walshe JM, Denduluri N. Systemic therapy for metastatic HER2-positive breast cancer. Semin Oncol 2020;47:259-69. [Crossref] [PubMed]
- Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14:461-71. [Crossref] [PubMed]
- Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:519-30. [Crossref] [PubMed]
- Kaufman PA, Hurvitz SA, O'Shaughnessy J, et al. Baseline characteristics and first-line treatment patterns in patients with HER2-positive metastatic breast cancer in the SystHERs registry. Breast Cancer Res Treat 2021;188:179-90. [Crossref] [PubMed]
- Cortés J, Kim S, Chung W, et al. Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. Presidential symposium 1. Ann Oncol 2021;32:S1283-S1346. [Crossref]
- Hurvitz SA, Kim SB, Chung WP, et al. Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03. 2021 San Antonio Breast Cancer Symposium; 2021 Dec 7-10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2022;82:Abstract nr GS3-01.
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Yan M, Bian L, Hu X, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res 2020;1:13. [Crossref]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732-42. [Crossref] [PubMed]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020;382:610-21. [Crossref] [PubMed]
- Hao C, Liu H. Current understandings and prospects of antibody-drug conjugates (ADCs) for the treatment of breast cancer: a narrative review. Transl Breast Cancer Res 2021;2:30. [Crossref]
- Wang J, Liu Y, Zhang Q, et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J Clin Oncol 2021;39:abstr 1022.
- Manich CS, O'Shaughnessy J, Aftimos PG, et al. LBA15 - Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Proffered Paper session - Breast cancer, metastatic. Ann Oncol 2021;32:S1283-S1346.
- Hu X, Zhang J, Ji D, et al. A phase 1 study of ARX788, a HER2-targeting antibody-drug conjugate, in patients with metastatic HER2-positive breast cancer. Cancer Res 2020;80:abstr P1-18-6.
- Hu X, Zhang J, Liu R, et al. Phase I study of A166 in patients with HER2-expressing locally advanced or metastatic solid tumors. J Clin Oncol 2021;39:abstr 1024.
- Li H, Zhang X, Xu Z, et al. Preclinical evaluation of MRG002, a novel HER2-targeting antibody-drug conjugate with potent antitumor activity against HER2-positive solid tumors. Antib Ther 2021;4:175-84. [Crossref] [PubMed]
Cite this article as: Yan Y, Li Q, Li J. Round table discussion: strategies for the treatment of HER2-positive advanced breast cancer in the rising age of antibody-drug conjugates. Transl Breast Cancer Res 2022;3:18.


