
Narrative review of progress in pathological diagnosis of breast cancer
Introduction
Breast cancer (BC) contains a spectrum of diseases with distinctive presentations, morphology, biology, and clinical phenotypes. Additionally, BC varies significantly in its biological behaviour and response to treatment. Combination of the traditional histopathological with the molecular types of BC allows more accurate prediction of its biological and clinical heterogeneity, and has been incorporated into pathology clinical practice as recommended by the Chinese Society of Clinical Oncology (CSCO) BC guidelines and guides the treatment of patients. With the increasing application of sophisticated molecular techniques, the accumulation of Next Generation Sequencing (NGS) big data, which may enable the continuous identification of new therapeutic targets and need for more accurate pathological classification systems that can accurately predict the outcome and response to treatment. This review outlines the latest advances in breast pathological detection and related biomarkers. I present the following article in accordance with the Narrative Review reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-11/rc).
Methods (Table 1)
Table 1
| Items | Specification |
|---|---|
| Date of Search (specified to date, month and year) | Feb 1, 2022 |
| Databases and other sources searched | PubMed, FDA and NMPA website |
| Search terms used (including MeSH and free text search terms and filters) | Histologic types, HER2-low, Ki67, ER, TILs, PD-L1 |
| Timeframe | 2000–2022 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | None |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | None |
HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PD-L1, programmed death protein-ligand 1.
Histological types and clinical significance
The 2019 World Health Organization (WHO) classification of breast tumours (5th edition) emphasizes the correlation of histologic types with prognosis and clinical management. The no special type (NST) of BC is the most common invasive patterns of BC and includes a spectrum of morphologic variants. In addition to the original special patterns of NST, including carcinoma with osteoclast-like giant cells, pleomorphism, choriocarcinomatous features, and melanotic features, special patterns such as invasive carcinoma with medullary features, some rare patterns of invasive carcinoma, and invasive carcinoma with neuroendocrine differentiation have been added (1). The new WHO classification highlights the typical morphological and molecular characteristics of invasive carcinoma with medullary features (NST), such as expansive growth, high histological grade, common necrosis, and abundant lymphocytic infiltration, with some patients carrying the BRCA1 mutation, most of which show the basal-like phenotype. Due to the poor reproducibility in diagnosis of this patient group, this group of tumours is no longer treated as a separate histological subtype and now comes under the broad category of invasive carcinoma (NST). Oncocytic, lipid-rich, glycogen-rich clear cell, and sebaceous carcinomas in the 2012 edition are now classified as special patterns of invasive carcinoma (NST) in the new edition due to their immunophenotypes, molecular genetic characteristics, and prognosis are unable to reach a consensus.
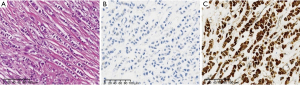
Lobular carcinoma accounts for approximately 5−15% of BC (2-4), and its pathological features are often characterized by the loss of tumour cell adhesion and loss of E-cadherin protein expression (Figure 1). The majority of classic invasive lobular carcinomas (ILCs) express both estrogen receptors (ER) and progesterone receptors (PR) but lack ERBB2 (human epidermal growth factor receptor 2, HER2) gene amplification/overexpression. Based on gene expression profiling, up to 85% of the population has been classified as classic subtype (5). Pleomorphic carcinoma is distinct from the classic type, exhibiting intermediate to high nuclear grade and is HER2-positive or triple negative breast cancer (5,6). The CDH1 mutation is the most common alteration in ILCs. However, approximately 17% of lobular carcinomas with typical morphologic features lack the CDH1 mutation and are positive for E-cadherin expression (7). At the 2021 San Antonio Breast Cancer Symposium, Professor Fatemeh Derakhshan (8) presented a study which demonstrated that CDH1 promoter methylation was presented in 56% of cases lacking CDH1 somatic mutations. Further 505 gene panel analysis showed that AXIN2 loss of function may be responsible for the loss of adhesion of lobular carcinoma cells that lack the CDH1 mutation (9). Therefore, further diagnosis of lobular carcinoma can be attempted in clinical practice using the AXIN2 antibody.
For special subtypes of BC, the new classification emphasizes paying attention to specific histological types and clinical treatment. For example, tubular carcinoma and invasive cribriform carcinoma have excellent prognosis and generally do not require adjuvant chemotherapy after surgery. And invasive micropapillary carcinomas are mostly the luminal subtype, which are highly susceptible to vascular tumour embolism and lymph node metastasis. Therefore, caution should be application when selecting neoadjuvant therapy. It is worth noting that the new classification adds some rare forms of triple-negative breast cancer (TNBC) which often have a relatively good prognosis. Mucinous cystadenocarcinoma is prevalent among postmenopausal women of Asian descent. Unlike mucinous carcinoma, mucinous cystadenocarcinoma tends to present with a triple-negative immunophenotype (1). Tall cell carcinoma with reversed polarity (9,10) is characterized by indolent biological behaviour and a histomorphology similar to that of the tall cell variant of papillary thyroid carcinoma but without the characteristic RET gene rearrangement and BRAF gene mutation in papillary thyroid carcinoma. Approximately 80% of cases harbour characteristic IDH2 hotspot mutations,secretory carcinoma accounts for 0.05% of all invasive BCs, occurring predominantly in women with a triple-negative or weakly ER/PR-positive immunophenotype (1). It carries a specific ETV6-NTRK3 gene fusion signature. At present, small-molecule inhibitors (Larotrectinib and Entrectinib) targeting neurotrophic tyrosine kinase (NTRK) 3 and other NTRK family members have been clinically applied, which have shown promising efficacy in secretory carcinomas (11).
Advances in HER2-low detection based on the clinical effects of antibody-drug conjugates (ADCs)
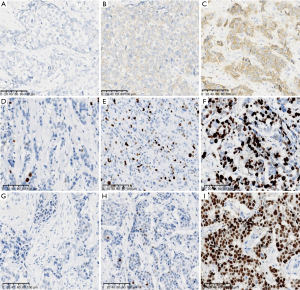
The HER2 is a tyrosine kinase receptor protein that belongs to the epidermal growth factor receptor (EGFR) family. The HER2/ErBb2/neu oncogene is located at chromosomal position 17q12. A proportion of 15% to 20% of breast cancers have protein overexpression/HER2 gene amplification (12), and they are associated with aggressive clinical behaviour and poor prognosis. In late 2019, two clinical studies on chemotherapy combined with anti-HER2 antibodies (13,14) demonstrated that trastuzumab (T-DXd or DS-8201a) and trastuzumab duocarmazine (SYD985) were highly efficacious in patients with a HER2 immunohistochemistry (IHC) 3+ or IHC 2+ but no amplification by in situ hybridization (ISH). The latest research (13) has shown that patients with BC having low levels of HER2 expression (IHC 1+, 2+ but ISH no amplification) can benefit from the use of ADCs. As a result, pathologists have incorporated the concept of the HER2-low category into their diagnostic practice. As the results of clinical trials for new treatment strategies emerged, the 2021 CSCO BC guidelines pioneered the concept of classifying HER2 expression into three categories. Moreover, France (15) updated its consensus on HER2 pathological detection of BC in November 2021, highlighting that HER2 expression is a continuous process, where HER2 1+ and 2+ with no amplification by ISH is classified as HER2-low, while HER2 2+ with ISH amplification and HER2 3+ is classified as HER2-high. During routine diagnosis, pathologists directly diagnose HER2 0 and 1+ as negative without distinction, whereas the French consensus (15) sets clear criteria for distinguishing and defining HER2 0 and 1. The interpretation criteria for ISH have not been updated, instead, groups 2, 4, and 5 of FISH are directly classified as HER2-Low. The emergence of the HER2-low definition poses a greater challenge for accurate HER2 interpretation (Figure 2A-2C). Overall, HER2-low BC is a separate entity in the heterogeneous population of HER2-negative tumours, with the proportion of patients with HER2-low BC accounting for more than half of all HER2-negative BCs (16,17).
Data from a survey by the College of American Pathologists (CAP) on HER2 cases in 1,452 pathology departments internationally was recently released in San Antonio 2021, with each lab providing 20 tissue specimens consisting of HER2 IHC 0, 1+ or 3+, but excluding HER2 2+ cases. The concordance for HER2 3+ exceeded 90%, while the concordance for HER2 0 and 1+ assessments was poor, at only 26% (18). Therefore, the Chinese Breast Pathology Group proposed to evaluate the concordance of different clones, strengthen training on HER2 interpretation. In addition, multiple tissues should be used as external controls during IHC assays, including HER2 IHC 1+, 2+, and 3+ samples. It should also be noted that for specific patterns of HER2 staining, invasive micropapillary carcinoma has a U-shaped staining pattern, while apocrine carcinomas and carcinomas with neuroendocrine features often exhibit cytoplasmic staining in HER2 IHC 1+ cases. These findings are prone to misclassification by pathologists as false positives, thus depriving patients of potential treatment. For HER2 IHC 2+ cases, analysis of HER2 gene status by ISH is required, and it is recommended that the number of cells analysed, the average HER2 and CEP17 copies per tumor cell, and the HER2/CEP17 ratio should be reflected in the report.
The introduction of the HER2-low category has prompted updates in the international guidelines for HER2 detection in BC, particularly with respect to the concept of clinical antibody-drug conjugates (ADC) treatment progress and low expression, the criteria for interpreting the three categories of HER2 expression, the enhancement of awareness and response to HER2 expression heterogeneity, the importance of HER2 mutation detection, and new methods of HER2 detection (especially artificial intelligence-assisted interpretation) which have reinforced the critical role of pathologists in assessing HER2 status in BC.
Clinical practice of updated international guidelines for Ki67 in BC
Ki67 IHC is currently the most commonly adopted method for detecting cell proliferation in BC and is mainly used to predict the prognosis of early-stage BC and the efficacy of chemotherapy or endocrine therapy. Additionally, it serves as a dynamic predictive factor before, during, and after neoadjuvant therapy (especially neoadjuvant endocrine therapy). However, it plays a limited role in treatment decision making due to its low clinical validity (19). The International Ki67 of Breast Cancer Working Group (IKWG) was founded in 2009 by the Breast International Group (BIG) and North American Breast Cancer Group (NABCG). After more than 10 years of reproducibility and accuracy studies, the IKWG released a Ki67 guidelines update on 28 December 2020 (20), which recommends the need to focus on preanalytical factors and staining procedures for Ki67 and the standardization or validation of scoring systems to improve Ki67 detection and interpretation accuracy in BC. Furthermore, it stresses that without further improvements in standardization, only Ki67 IHC findings of ≤5% and ≥30% can be classified as low and high expression in clinical practice, respectively (Figure 2D-2F). Hence, the IKWG recommends that unless a particular centre has calibrated assay performance for a specific intended use and clinical prognosis, only Ki67 IHC findings of ≤5% or ≥30% can be used to guide the patient’s treatment decisions. Ki67 IHC has a weak to moderate correlation with multigene molecular assays, and there is a high variability in the correlation between the expression levels of specific proliferation genes in gene profiling and clinical prognosis. However, inter-observer/inter-laboratory variability is relatively large in the 5−30% range, and multigene expression profiling is still recommended according to the American Society of Clinical Oncology (ASCO) guidelines (19). Thus, the role of Ki67 in clinical prediction is complex. Should a dichotomous approach or a continuous score be adopted to enhance reproducibility in pathology reports? If continuous scoring is used, how should the cut-off value be chosen? These questions remain inconclusive.
Following the successive approval of Abemaciclib in several countries for the adjuvant intensive treatment of early-stage high-risk BC, the results of the MonarchE study (21) demonstrated that by setting the cut-off value at 20%, patients with Ki67 ≥20% showed greater absolute improvements in terms of invasive disease-free survival (IDFS). This suggests that the Ki67 index can be combined with clinicopathological features (e.g., lymph node involvement, tumour size, and histological grade) for the selection of patients at high risk for recurrence, thereby screening for groups that will gain superior benefits from adjuvant Abemaciclib. Furthermore, the National Comprehensive Cancer Network (NCCN) guidelines and the Food and Drug Administration (FDA) both recommend conducting Ki67 testing if adjuvant intensive therapy with Abemaciclib is planned. In 2022, the NCCN proposed that for high-risk patients with hormone receptor (HR)-positive HER2-negative BC (i.e., ≥4 positive lymph nodes or 1−3 positive lymph nodes with at least one of the following: grade 3 lesion, tumour size ≥5 cm, and Ki-67 score ≥20%), adjuvant Abemaciclib may be considered for 2 years after surgery. A clinical trial in San Antonio 2021 revealed that, HR-positive/HER2-negative patients with Ki67 ≥20% at baseline, following short-term Palbociclib and Letrozole neoadjuvant endocrine therapy (ET) and sequential biopsies for the dynamic detection of Ki67, enabled a large percentage of patients to achieve pathological or molecular downstaging.
Weak positive ER and heterogeneity should be concerned in BC management
The ASCO/CAP guidelines for ER and PR detection were first published in 2010 (22), which defined 1−100% ER or PR as positive, with recommendation of 1% as the cut-off value for positive ER expression and ET. In 2020, the ASCO/CAP updated the guidelines for ER detection (23), and the expert panel suggested that although 1% is recommended as the cut-off value for positive ER expression and ET, there is a limited data on the benefits for BC in the 1−10% range of ER expression, and a new reporting category should be used: weakly ER-positive.
ER expression distribution is characteristic, with most cases falling in the negative and >90% positive categories. While patients with weakly ER-positive are uncommon (only 2−3%), it is now generally accepted that the benefits of ET are unclear for this subgroup, and is a heterogeneous spectrum. Therefore, the clinical prognosis and biological behaviour of such patients are similar to ER-negative cases, and their treatment options should be selected based on clinical, pathological, and molecular features. Furthermore, some clinical and pathological experts have recommended that this weakly ER-positive subgroup should be included in TNBC clinical trials.
BC is a heterogeneous disease that exhibits temporal and spatial heterogeneity. Temporal heterogeneity refers to the differences in primary tumours at different time periods during tumour progression or differences in molecular types and biological behaviour between primary and metastasis lesion. In clinical practice, up to 15−20% of patients have changes in ER status between primary and metastasis lesion, thus indicating the presence of temporal heterogeneity in ER expression. A study of San Antonio in 2021 revealed large-scale molecular differences between ER positive and negative conversions, suggesting the presence of different cellular origins at the primary tumour (i.e., the luminal and basal-like subtypes), especially in patients with BC having mixed molecular subtypes. Thus, there may be a subtype of patients with BC having an ER-positive rate below 100% with both luminal and basal-like cells that may still existing after adjuvant therapy and contribute to recurrence. Spatial heterogeneity refers to differences between cells at different tissue sites in the same tumour. Studies have shown (24) that intra-tumour heterogeneity in ER expression can be used as an independent predictor of sensitivity to ET, and long-term survival is significantly lower in patients with highly heterogenous ER positivity (Figure 2G-2I).
Whether tumor infiltrating lymphocytes is recommended in routine pathological reports
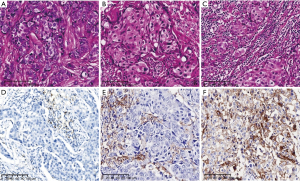
Tumor infiltrating lymphocytes (TILs) refer to the heterogeneous population of lymphoid cells (predominantly lymphocytes) found in tumour cell nests and tumour stroma, which are involved in the immune response and regulation in tumour immune mechanisms. TILs are located around tumour tissues which can kill tumour cells and reduce tumour metastasis(25). In the international guidelines for TILs detection issued by the TILs International Working Group in 2014 (26), a methodology was recommended, which was based on several large clinical trials and basic studies on the histological assessment of TILs. To facilitate the use of TILs as biomarkers in basic and clinical research (i.e., as stratification or adjustment factors), which would enable their eventual application in diagnostic practice, this methodology involved the visual assessment of haematoxylin and eosin (H&E) sections to determine the percentage of TILs in primary tumour specimens. This assessment mainly focused on: delineating the tumour area for assessment; how to score TILs level (Figure 3A-3C); and the significance of TILs in clinical treatment. Since then, the Working Group has been constantly striving to fully verify the analytical and clinical validity of assessment methodology and has established a dedicated TILs website where the assessment procedure is described in detail. At the 17th St. Gallen International Breast Cancer Congress (SG-BCC 2021), 61% of experts voted against the inclusion of TILs evaluation in routine pathology practice. Anyhow, based on the BIG 2-98, ECOG 2197, and 1199 clinical trials (27-29), patients with a higher number of stromal TILs at diagnosis showed better outcomes after anthracycline-based adjuvant chemotherapy, while the prognostic value of TILs in TNBC can be considered as level I evidence. TILs have not been adopted as prognostic biomarkers for chemotherapy owing to the lack of prognostic information in chemotherapy-naive patients with primary TNBC. Based on the FinHER clinical trial, higher TILs in patients with HER2-positivity resulted in better responses to trastuzumab therapy, while recent data from the N9831 trial indicated that tumours defined as ‘immune-enriched’ responded more effectively to trastuzumab therapy. Although these findings do not affect the use of trastuzumab in newly diagnosed HER2+ BC, they suggest a potential mechanism of action for trastuzumab-based therapy. TILs can predict the efficacy of trastuzumab in patients with TNBC and HER2-positivity. Thus, it will soon be incorporated into the American Joint Committee on Cancer (AJCC) staging and prognosis system. Furthermore, the Annals of Oncology just published a review in December (30), which mainly emphasized that TILs are significantly associated with better prognosis in TNBC and HER2+ BC (level IB evidence) and have been included in some international BC guidelines and routine pathology reports. The review also recommended using the 30% cut-off value as a suitable reference for whether to perform adjuvant chemotherapy in stage I TNBC. As the first biological prognostic marker for TNBC, TILs can be used for the patient’s AJCC TNM staging, while TILs expression can also facilitate the clinical screening of patients with BC who will gain the greatest benefit from programmed death protein-1/programmed death protein-ligand 1 (PD-1/PD-L1) therapy. In patients with advanced TNBC and HER2+ BC, a cut-off value in TILs of 5% or 10% with concurrent PD-L1 expression is defined as an ‘immune-enriched’ tumour.
Understanding the companion diagnostic concepts of PD-L1 for breast cancer immunotherapy
Based on the IMpassion 130 and Keynote-355 trials, the PD-L1 (VENTANA SP142) immune cell scoring system and the PD-L1 (DAKO 22C3) combined positive scoring system for tumour cells and immune cells, and they were approved as companion diagnostics for PD-L1 immune checkpoint inhibitors. Although the two clinical trials had similar outcomes and both showed good clinical benefit, they had slightly different positive threshold, PD-L1 testing platforms and scoring methods. Thus, PD-L1 antibodies and score methods, their clinical applications are different. Their results are cannot replace each other (Figure 3D-3F).
Among the clinical trials of immunotherapy, the histological types of TNBC were not subdivided; however, TNBC includes a biologically diverse spectrum of low and high grades. For example, most cases of salivary gland-type tumours have a good prognosis and indolent biological behaviour which qualified as low grade. In addition, fibromatous-like metaplastic carcinomas and adenosquamous carcinomas that are also classified as low grade. The biological behaviour of high-grade metaplastic carcinomas resembles as sarcomas, and their immune microenvironments are mostly regarded as immune deserts. TNBC has high genomic instability and a high mutation rate, rendering it the most immunogenic subtype of BC due to its ability to produce a relatively large amount of neoantigens. Based on genomic and transcriptomic data, TNBC has also been previously classified into the following six subtypes (31): basal-like 1, basal-like 2, mesenchymal-like, mesenchymal stem-like, luminal androgen receptor, and immunomodulatory. Therefore, in clinical practice, the molecular heterogeneity of TNBC needs to be considered, as different molecular subtypes have different immune microenvironments.
Compared to ER and HER2, PD-L1 expression is more spatiotemporally heterogeneous. Based on data from the IMpassion 130 trial, PD-L1 expression levels are lower in metastases, with lower metastasis rate in liver than lymph node (32). This suggests that we must re-evaluate the expression of PD-L1 in biopsies of distant metastases to optimize the clinical application of PD-L1. Therefore, in practice, spatial and temporal heterogeneity must be considered in PD-L1 detection. Providing a true reflection of the patient’s PD-L1 expression would be very helpful in screening patients suitable for immunotherapy and comprehensively improving patient survival.
Conclusions
The histological types and molecular pathological markers of BC are closely related to its treatment and prognosis. The 5th edition WHO classification of Breast tumours more emphasis on the clinical significance of histological types. The consensus on the testing of biomarkers for BC has also become more precise, not only suggesting that the primary and metastases of BC be detected for the expressions of ER, PR, and HER2, but also introducing the concept of ER and HER2 low-expressions. In addition, the Ki67 proliferation index plays an important role in the expected therapeutic effect. With the gradual rise of immunotherapy, treatments based on PD-1/PD-L1 immune checkpoint inhibitors have achieved some results in clinical efficacy for BC, while studies related to TILs are gradually gaining more attention. Patients with TNBC and HER2-positive BC are characterized by a high proportion of TILs, and TILs may be a better predictor of chemotherapy response and survival outcomes. With the continued exploration of immunotherapy for TNBC, relevant medicine is gradually moving from preclinical trials to clinical practice. There are many other immunohistochemical staining indexes cannot be elaborated, such as progesterone receptor, and androgen receptor, CK5/6, etc. These markers also play a certain role in diagnosis, typing and treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-11/rc).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-11/coif). YL serves as an unpaid editorial member of Translational Breast Cancer Research from May 2021 to April 2023. The author has no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO classification of tumours editorial board. Breast tumours. Lyon: IARC Press, 2019. 5th ed. vol 2.
- Ellis IO, Galea M, Broughton N, et al. Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 1992;20:479-89. [Crossref] [PubMed]
- Li CI, Anderson BO, Daling JR, et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 2003;289:1421-4. [Crossref] [PubMed]
- Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer 2005;93:1046-52. [Crossref] [PubMed]
- Ciriello G, Gatza ML, Beck AH, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015;163:506-19. [Crossref] [PubMed]
- Desmedt C, Zoppoli G, Gundem G, et al. Genomic Characterization of Primary Invasive Lobular Breast Cancer. J Clin Oncol 2016;34:1872-81. [Crossref] [PubMed]
- Grabenstetter A, Mohanty AS, Rana S, et al. E-cadherin immunohistochemical expression in invasive lobular carcinoma of the breast: correlation with morphology and CDH1 somatic alterations. Hum Pathol 2020;102:44-53. [Crossref] [PubMed]
- Fatemeh D, Higinio D, Da Cruz Paula A, et al. Genetic and epigenetic basis of lobular carcinomas lacking CDH1 alterations. Cancer Res 2022;82:PD14-03.
- Lozada JR, Basili T, Pareja F, et al. Solid papillary breast carcinomas resembling the tall cell variant of papillary thyroid neoplasms (solid papillary carcinomas with reverse polarity) harbour recurrent mutations affecting IDH2 and PIK3CA: a validation cohort. Histopathology 2018;73:339-44. [Crossref] [PubMed]
- Foschini MP, Asioli S, Foreid S, et al. Solid papillary breast carcinoma resembling the tall cell variant of papillary thyroid neoplasms: a unique invasive tumor with indolent behavior. Am J Surg Pathol 2017;41:887-95. [Crossref] [PubMed]
- Shukla N, Roberts SS, Baki MO, et al. Successful Targeted Therapy of Refractory Pediatric ETV6-NTRK3 Fusion-Positive Secretory Breast Carcinoma. JCO Precis Oncol 2017; [Crossref] [PubMed]
- Modi S, Park H, Murthy RK, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol 2020;38:1887-96. [Crossref] [PubMed]
- Hammond ME, Hayes DF, Wolff AC. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol 2011;29:e458. [Crossref] [PubMed]
- Xu Z, Guo D, Jiang Z, et al. Novel HER2-Targeting Antibody-Drug Conjugates of Trastuzumab Beyond T-DM1 in Breast Cancer: Trastuzumab Deruxtecan(DS-8201a) and (Vic-)Trastuzumab Duocarmazine (SYD985). Eur J Med Chem 2019;183:111682. [Crossref] [PubMed]
- Franchet C, Djerroudi L, Maran-Gonzalez A, et al. 2021 update of the GEFPICS' recommendations for HER2 status assessment in invasive breast cancer in France. Ann Pathol 2021;41:507-20. [Crossref] [PubMed]
- Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021;7:1. [Crossref] [PubMed]
- Tarantino P, Hamilton E, Tolaney SM, et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J Clin Oncol 2020;38:1951-62. [Crossref] [PubMed]
- Aileen I, Matthew L, Andrew B, et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol. ; [Crossref]
- Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011;103:1656-64. [Crossref] [PubMed]
- Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 2021;113:808-19. [Crossref] [PubMed]
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 2021;32:1571-81. [Crossref] [PubMed]
- Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784-95. [Crossref] [PubMed]
- Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol 2020;38:1346-66. [Crossref] [PubMed]
- Lindström LS, Yau C, Czene K, et al. Intratumor Heterogeneity of the Estrogen Receptor and the Long-term Risk of Fatal Breast Cancer. J Natl Cancer Inst 2018;110:726-33. [Crossref] [PubMed]
- Razazan A, Behravan J. Single peptides and combination modalities for triple negative breast cancer. J Cell Physiol 2020;235:4089-108. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumorinfiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013;31:860-7. [Crossref] [PubMed]
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes is prognostic and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014;25:1544-50. [Crossref] [PubMed]
- Adams S, Demaria S, Goldstein L, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes (TILs) in Triple Negative Breast Cancers (TNBC) from two Phase III Randomized, Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959-66. [Crossref] [PubMed]
- Loi S, Michiels S, Adams S, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol 2021;32:1236-44. [Crossref] [PubMed]
- Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67. [Crossref] [PubMed]
- Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:44-59. [Crossref] [PubMed]
Cite this article as: Liu Y. Narrative review of progress in pathological diagnosis of breast cancer. Transl Breast Cancer Res 2022;3:16.


