
Reduction in tumor grade and Ki-67 in postmenopausal patient with node-positive invasive ductal carcinoma following combination hormone replacement therapy cessation: a case report
Introduction
Hormone replacement therapy (HRT) has gained popularity among patients for its alleviation of menopausal symptoms and known efficacy for treatment of osteopenia, osteoporosis, vasomotor symptoms, obesity, vaginal atrophy and dryness (1,2). However, the treatment is not without its risks, with increased associations with major cardiovascular and thromboembolic events, and endometrial and breast cancers (2,3). HRT is a known risk factor for development of breast cancer, particularly with combination therapies of estrogens and progestins and with extended durations of use. Estrogen/progestin HRT is associated with increased density changes on mammography, leading to more difficult diagnosis of small cancers and delay in treatment (4). Further, combination therapies have been shown to increase incidence of breast cancers with an unknown impact on prognosis or mortality (5,6). Few studies have examined any potential effect HRT administration or withdrawal may have on existing breast cancers between time of biopsy and surgical intervention. We present herein the first case of a 64-year-old female with node-positive invasive ductal carcinoma diagnosed in the setting of longstanding HRT that was withdrawn after time of biopsy before definitive surgical management, demonstrating relative reduction in tumor proliferation by 28.6%. We present the following case in accordance with the CARE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-26/rc).
Case presentation
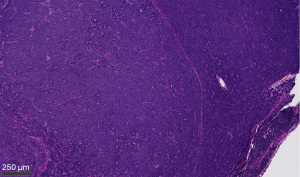
On January 22, 2021, a 64-year-old female presented with a new palpable mass in the upper outer quadrant of the left breast. She had no breast related symptoms with the exception of the palpable left breast mass. The patient was on HRT with an estrogen patch and progesterone, which she had been taking for almost 15 years for menopausal symptoms, including hot flashes and insomnia. Family history was positive for breast cancer in her paternal aunt, melanoma in her mother, and squamous cell carcinoma in her sister. On exam, left breast mass was palpable with no overlying skin changes, nipple retraction, or nipple discharge, and a small, mobile lymph node without matting was palpated in the left axilla. She underwent diagnostic mammogram, which demonstrated a 1.2 cm mass corresponding to the area of palpable concern, as well as a partially visualized enlarged left axillary node. No other masses, suspicious calcifications, or areas of architectural distortion were noted. Targeted ultrasound of the left breast and axilla demonstrated the 1.1 cm irregular mass at the 2 o’clock position and an enlarged lymph node with single cortical thickness measuring 7 mm (BI-RADS 4). Ultrasound-guided biopsy was performed, and pathology showed poorly differentiated invasive ductal carcinoma with Nottingham score of 8 (Figure 1). The tumor was estrogen receptor (ER) positive (100%), progesterone receptor (PR) positive (100%), and human epidermal growth factor receptor (HER2/neu) negative, with proliferation index marker Ki-67 of 70% (Figure 2). Lymph node pathology showed metastatic, poorly differentiated carcinoma (Figure 3).


The patient subsequently presented for surgical consultation. She was instructed to discontinue use of her HRT in the setting of breast carcinoma with ER and PR positivity. Staging CT showed left axillary lymphadenopathy with no other evidence of metastatic disease in the chest, abdomen, and pelvis. Bone scan showed slightly increased asymmetric radiotracer uptake in the L4 vertebral body and right hip correlating with degenerative disease with low suspicion for metastasis. Genetic testing for BRCA1 and BRCA2 was found to be negative. Given these findings, it was determined that the patient had a clinical prognostic stage of IIA (T1c, N1, M0). Per tumor board recommendations, the decision was made to proceed with upfront surgery and with possible adjuvant chemotherapy pending final pathology and Oncotype score. Sixty-eight days following initial presentation, she underwent left breast lumpectomy and left axillary lymph node dissection with oncoplastic closure of lumpectomy cavity. Surgical specimen of the left breast mass demonstrated a magnetic seed and coil clip located within the specimen. Final surgical pathology of the left breast lumpectomy specimen revealed a 1.4 cm grade 2 invasive ductal carcinoma with a Nottingham histologic score of 7 and Ki-67 of 50% as well as focal intermediate nuclear grade ductal carcinoma in situ (DCIS) with solid and cribriform patterns comprising less than 5% of the tumor (Figures 4,5). Margins were noted to be negative. Left axillary contents revealed metastatic ductal carcinoma to three of 18 lymph nodes. Postoperatively, the patient was referred to radiation oncology for adjuvant whole breast radiation therapy. The patient was pleased with the care she received. The patient has remained recurrence-free at 1.3 years postoperatively with continued follow-up. This case report conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.


Discussion
While there have been many proposed benefits of HRT in postmenopausal women, such as fracture risk reduction, vasomotor symptom control, and relief for vaginal dryness and atrophy, the demonstrated risks continue to be a true barrier to recommending HRT for all populations. In the Women’s Health Initiative’s randomized controlled trial from 1993–1998 with 16,608 women aged 50–79 that measured the effect of HRT on different outcomes, there was overwhelming evidence of breast cancer harm induced by estrogen-progestin combination HRT (z score of 2.56 for 5-year trend) outweighing potential benefits against fractures and colon cancer. This combination HRT arm of the study was discontinued immediately, while the unopposed estrogen HRT arm was continued as the balance of risks and benefits was unknown. This trial demonstrates the association between combination HRT and breast cancer development in this age group with a large and representative sample size but goes one step further to analyze the risks and benefits (7). With our patient’s known ER/PR positive invasive carcinoma and demographic profile fitting the cohort of this study, the evidence is overwhelmingly in support of combination HRT discontinuation in her case in regard to both her existing cancer but also mitigation of future breast cancers especially given her younger age.
While estrogen-progestin combination HRT has been associated with increased breast cancer risk, few studies have elucidated a true relationship between estrogen-only therapy and cancer risk. In an extended follow-up of the Women’s Health Initiative randomized control trial examining conjugated equine estrogen only versus placebo, overall rates of breast cancers arising in the HRT group were actually significantly lower compared to placebo [0.27% vs. 0.35%; HR, 0.77; 95% CI: 0.62–0.95; P=0.02] though this was only true for invasive ductal carcinomas, not lobular, when analysis was stratified further (8). However, the original trial of the estrogen-only arm was ended prematurely when the evidence strongly suggested high risk for stroke and venous thromboembolism in the HRT group compared to placebo, determining that the risk for ischemic events was too great to outweigh the potential breast cancer benefit. This study highlights the need for investigation of estrogen-only HRT and its role in breast cancer development, particularly in patients with preexisting breast cancers, to determine whether such therapies are appropriate for populations like that of our patient. Further, the study demonstrates the very real risks associated with exogenous hormone administration and that risk mitigation is crucial when selecting the appropriate modality for treatment of menopause symptoms.
Though it has been suggested that there is a meaningful reduction in the effects of HRT immediately after cessation, it may take up to 5 years to see complete dissipation of the lasting effects (9). Multiple studies have shown statistically significant reduction in Ki-67 and tumor size on imaging of hormone receptor-positive tumors after HRT cessation (10,11). Based on preoperative imaging studies and postoperative pathologic examination, our patient’s tumor increased slightly in size from 1.2 cm on ultrasound to 1.4 cm on gross inspection even after HRT cessation. This growth is consistent with the initial high-grade nature of the poorly differentiated tumor and high overall average tumor proliferation index marker (Ki-67) >30%. However, the final pathology report after surgical excision revealed a small reduction in Nottingham score from 8 to 7, downgrade of the tumor from poorly differentiated to moderately differentiated, and a concomitant decrease in Ki-67 from 70% at time of biopsy to 50% at time of surgical excision, which were about 2 months apart. Though decreased, the final Ki-67 was still considered to be in the high range (>30%). While a more dramatic decrease was expected clinically, the lasting effects of HRT require additional time to fully dissipate. Additionally, studies suggest neoadjuvant endocrine therapy with aromatase inhibitors results in significantly greater reduction in Ki-67, a treatment option that might be considered in patients like ours (12). The period of 68 days between presentation and surgery is in accordance with current clinical guidelines to pursue surgery within 90 days of diagnosis. Given the lack of literature on the optimal period between surgery and HRT cessation, the patient’s surgical timeline was not extended as is seen in patients who receive neoadjuvant endocrine therapy. Current clinical trials suggest, loosely centered on preoperative tamoxifen and chemotherapy recommendations, to pursue a neoadjuvant endocrine therapy duration of at least three months, with no formal recommendations to extend this timeline (13-15). Future studies are necessary to investigate this topic further to identify the optimal treatment duration for neoadjuvant endocrine therapy. With Ki-67 emerging as an important prognostic biomarker in breast cancer, the relative reduction in Ki-67 by 28.6% represents a meaningful decrease in tumor cell proliferation and alludes to a clinical benefit of HRT cessation in this patient with known invasive carcinoma and node-positive disease. Recent clinical trials identified Ki-67 as a valuable prognostic marker in those with high baseline Ki-67. In 4,480 patients with hormone receptor-positive breast cancer, following 2 weeks of neoadjuvant treatment with an aromatase inhibitor in patients with high baseline Ki-67, the POETIC trial observed 5-year recurrence risks of 8.4% and 21.5% among patients with low and high Ki-67 at 2 weeks, compared to just 4.3% in patients with low baseline and 2-week Ki-67 (16). Similarly, the P024 trial demonstrated that tumor cell aromatase expression was negatively correlated with Ki-67 levels, alluding to the usefulness of aromatase expression as a favorable clinical and prognostic biomarker, perhaps due to more significant aromatase inhibition in patients with lower Ki-67 (17).
Strengths of this study include the quantitative evaluation of the perceived benefit of decreased tumor proliferation with HRT cessation as well as the high relative reduction in Ki-67 observed, alluding to a meaningful clinical outcome. As with all case reports, the major limitation of this study is the sample size of one, requiring further investigation into this subpopulation of breast cancer patients currently receiving HRT. Beyond the known benefit of mitigating future cancer development, further investigations are necessary to determine the clinical applicability of combination HRT cessation in patients with preexisting breast malignancies.
Cessation of combination HRT in a 64-year-old patient with known node-positive invasive ductal carcinoma resulted in relative reduction of Ki-67 by 28.6% and reduction in tumor grade. Discontinuation of HRT may be indicated in patients with preexisting tumors, particularly in those with ER/PR-positive and rapidly proliferating tumors.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-26/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-26/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This case report conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sørensen MB, Rosenfalck AM, Højgaard L, et al. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res 2001;9:622-6. [Crossref] [PubMed]
- Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev 2017;1:CD004143. [Crossref] [PubMed]
- Vickers MR, MacLennan AH, Lawton B, et al. Main morbidities recorded in the women's international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. BMJ 2007;335:239. [Crossref] [PubMed]
- Byrne C, Ursin G, Martin CF, et al. Mammographic Density Change With Estrogen and Progestin Therapy and Breast Cancer Risk. J Natl Cancer Inst 2017;109:djx001. [Crossref] [PubMed]
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ 2020;371:m3873. [Crossref] [PubMed]
- Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women's Health Initiative Randomized Clinical Trials. JAMA 2020;324:369-80. [Crossref] [PubMed]
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321-33. [Crossref] [PubMed]
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial. Lancet Oncol 2012;13:476-86. [Crossref] [PubMed]
- Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 1997;350:1047-59. Erratum in: Lancet 1997;350:1484. [Crossref] [PubMed]
- Burnside ES, Trentham-Dietz A, Kelcz F, et al. An Example of Breast Cancer Regression on Imaging. Radiol Case Rep 2006;1:27-37. [Crossref] [PubMed]
- Prasad R, Boland GP, Cramer A, et al. Short-term biologic response to withdrawal of hormone replacement therapy in patients with invasive breast carcinoma. Cancer 2003;98:2539-46. [Crossref] [PubMed]
- Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res 2005;11:951s-8s. [Crossref] [PubMed]
- Bleicher RJ. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann Surg Oncol 2018;25:2829-38. [Crossref] [PubMed]
- Carpenter R, Doughty JC, Cordiner C, et al. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat 2014;144:569-76. [Crossref] [PubMed]
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Smith I, Robertson J, Kilburn L, et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): an open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol 2020;21:1443-54. [Crossref] [PubMed]
- Ellis MJ, Miller WR, Tao Y, et al. Aromatase expression and outcomes in the P024 neoadjuvant endocrine therapy trial. Breast Cancer Res Treat 2009;116:371-8. [Crossref] [PubMed]
Cite this article as: King CA, Masanam MK, Maini AS, Merritt CM, Fan KL, Greenwalt IT. Reduction in tumor grade and Ki-67 in postmenopausal patient with node-positive invasive ductal carcinoma following combination hormone replacement therapy cessation: a case report. Transl Breast Cancer Res 2022;3:29.


