
Efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive advanced metastatic breast cancer: results from a randomized, open-label, multicenter, phase II bridging study
Introduction
Breast cancer is the most common cancer among Chinese women, ranking 1st in incidence (59.0/100,000 population) and 4th in mortality (16.6/100,000 population) among female malignant tumors in 2020 (1). Although early diagnosis and treatment can lower breast cancer-related mortality, the incidence of breast cancer is still on the rise (1,2), and advanced breast cancer remains largely incurable, with a 5-year survival of 20% and a median survival between 2–3 years (3-5). The main treatment goals of advanced breast cancer treatment are to relieve symptoms, improve quality of life and prolong patient survival (3).
Around 20% of breast cancers are human epidermal growth factor receptor 2 (HER2)-positive (6), which has more aggressive clinical behavior and worse patient survival outcomes as compared with estrogen receptor (ER) positive, HER2 negative breast cancer (7,8). In line with international practices, various anti-HER2 treatments including trastuzumab based anti-HER2 therapies (such as trastuzumab-pertuzumab-taxane), tyrosine kinase inhibitors (TKIs; such as pyrotinib and lapatinib) and ado-trastuzumab emtansine (T-DM1) have been approved in China for first- and second-line treatments of HER2-positive advanced metastatic breast cancer (MBC) (5,6). However, drug resistance to these treatments remains a major challenge for HER2-positive MBC patients, and most patients eventually experience disease progression (9,10). These patients face limited treatment options, as currently there is no recommendation of standard late-line treatment (10).
Margetuximab is a human/mouse chimeric anti-HER2 immunoglobulin G1 (IgG1) monoclonal antibody based on the murine precursor of trastuzumab (11). In addition to having similar epitope specificity and antiproliferative properties to trastuzumab, the optimized Fc domain of margetuximab has enhanced binding affinity for activating Fcγ receptor CD16A (including the low-affinity variant of CD16A, CD16A-158F) and reduced affinity for inhibitory Fcγ receptor CD32B, resulting in stronger antibody-dependent cellular cytotoxicity and thus greater anti-tumor activity (11-13).
In the international, multicenter, randomized, open-label phase III SOPHIA study (NCT02492711), a total of 536 HER2-positive MBC patients with disease progression on two or more prior anti-HER2 therapies and one to three lines of therapy for metastatic disease were randomized 1:1 to receive margetuximab or trastuzumab, both with chemotherapy (12). The results showed that margetuximab significantly improved the median progression-free survival (mPFS) over trastuzumab [5.8 vs. 4.9 months; hazard ratio (HR) =0.76; 95% confidence interval (CI): 0.59–0.98; P=0.03] with a largely comparable safety profile (12). As such, margetuximab was approved by the U.S. Food & Drug Administration for pretreated HER2-positive MBC in December 2020 (14), and has subsequently been recommended by the U.S National Comprehensive Cancer Network and the European Society for Medical Oncology for the same indication (5,15).
However, patients of the SOPHIA trial were mostly from Europe and North America, and none was from China. Therefore, although margetuximab may be a valuable addition to the treatment options for Chinese patients with pretreated HER2-positive MBC patients, it is uncertain whether population differences between Western and Chinese patients could affect the efficacy and safety of margetuximab. As such, a randomized, open-label, multicenter, phase II, bridging study was conducted to evaluate the efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive MBC, and to determine whether the results are consistent with the clinical benefit observed in the global population of SOPHIA. We present the following article in accordance with the CONSORT reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-35/rc).
Methods
Study design and participants
This is a randomized, open-label, multicenter, phase II, bridging study evaluating the efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive MBC (ClinicalTrials.gov Identifier: NCT04262804). Information of patient demographics (such as date of birth and weight) and baseline characteristics [such as Eastern Cooperative Oncology Group (ECOG) performance status and HER2 status] were collected during screening or routine clinical assessment if the routine assessment occurred within the protocol-specified screening windows (within 7 or 28 days prior to randomization, depending on the item collected). Eligible patients were Chinese patients (from 33 study sites in Chinese mainland, Hong Kong, and Taiwan, listed in Table S1) aged 18 years or older, with histologically confirmed HER2-positive MBC [HER2-positivity was defined as an immunohistochemical (IHC) staining score of 3+ or in situ hybridization (ISH) amplified by either fluorescence or chromogenic ISH as assessed by pathology department at the participating sites or qualified central laboratories which met the national or regional standards] and ECOG performance status of 0 or 1. Patients must have been treated for metastatic disease with two or more lines of anti-HER2 therapies (must include trastuzumab) and no more than three lines of antitumor therapy (including anti-HER2 therapies or chemotherapies), with disease progression during or after the most recent line of therapy. Patient must not have leptomeningeal metastases or ongoing, symptomatic brain metastases. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice Guidelines. The study protocol was approved by institutional review board/independent ethics committee at each participating site. All patients provided written informed consent before participating in the study.
Randomization and masking
Patients were randomly assigned (1:1) to margetuximab plus chemotherapy or trastuzumab plus chemotherapy by stratified block randomization with a block size of four, via an interactive web-based response system with a dynamic randomization list. Stratification factors included number of metastatic sites (≤2, >2) and chemotherapy choice, which could be capecitabine, vinorelbine, gemcitabine, or eribulin (only available for patients enrolled under protocol versions 1.0 and 2.0 as protocol was then updated to better reflect the treatment landscape in China). The randomization sequence was generated by the service provider of the interactive web-based response system. The investigators registered patients at each study center via the web-response system and assigned them to the treatment arms based on the randomization sequence directly obtained from the system. The web-response system ensured that the container sequence was concealed. The study was open label for participants and investigators but sponsor-blinded. To prevent observer bias, key efficacy endpoints were assessed by blinded independent central review (BICR), in which all imaging examinations were reviewed centrally by independent radiologists blinded to the patients’ treatment assignments.
Procedures
Each treatment cycle was 21 days. Margetuximab or trastuzumab was given intravenously on the first day of each cycle. Margetuximab was administered at 15 mg/kg over at least 120 minutes. Premedication (standard doses of acetaminophen or ibuprofen, diphenhydramine, ranitidine, and dexamethasone, or equivalents) should be given within 30 minutes before patients received margetuximab during treatment cycle 1 and in subsequent treatment cycles if infusion-related reactions (IRRs) occurred. Trastuzumab was given intravenously at the loading dose of 8 mg/kg over at least 90 minutes in treatment cycle 1 and at 6 mg/kg over at least 30 minutes in subsequent treatment cycles. Chemotherapy was administered first when given on the same day as margetuximab or trastuzumab. Capecitabine was given orally twice a day from day 1 to day 14 of each treatment cycle at 1,000 mg/m2, followed by 7 days off. Vinorelbine was given intravenously on days 1 and 8 of each treatment cycle at 25–30 mg/m2. Gemcitabine was given intravenously on days 1 and 8 of each treatment cycle at 1,000 mg/m2. To ensure treatment adherence, the study drug was administered to patients by trained medical personnel at the participating sites under staff supervision, and records of dosing and administration of all administered therapies were documented.
Patients received treatment until one of the following occurred: disease progression, intolerable toxicity, withdrawal of consent, initiation of follow-up antitumor treatment, loss to follow-up, or death. Disease assessment was conducted at baseline and after every two treatment cycles (6 weeks ± 7 days) and disease progression was assessed by BICR based on the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1). Safety was assessed at each visit, where investigators assessed the severity of treatment-emergent adverse events (TEAEs) and causality. Left ventricular ejection fraction (LVEF) was also monitored every 6 weeks. After the end of treatment, patients were followed-up for subsequent anti-tumor treatment and survival every three months until death, loss to follow-up, or end of the study, whichever occurred first. The study was conducted by trained personnel, and investigators at all participating sites followed the prespecified protocol strictly to ensure standardized procedures across centres.
Outcomes
The primary endpoint was PFS (defined as the time from randomization to disease progression or death from any cause, whichever occurred first) as assessed by BICR, which served to ensure blinded and standardized outcome assessment across centers. Secondary efficacy endpoints included overall survival (OS), investigator-assessed PFS, objective response rate (ORR; defined as the percentage of patients with BICR-assessed, confirmed complete or partial response), duration of response (DoR; defined as the time from the first documented complete or partial response to disease progression or death, whichever occurred first), and clinical benefit rate (CBR; defined as the percentage of patient with BICR-assessed confirmed complete or partial response, or stable disease lasting for ≥6 months). For safety, the incidence and severity of TEAEs evaluated based on the Common Terminology Criteria for Adverse Events, version 4.03 (CTCAE v4.03) were reported.
Statistical analysis
This is a bridging study aiming to demonstrate the consistency of margetuximab’s treatment effect on PFS between this study and the global phase III SOPHIA study. The consistency criterion for bridging success was defined as observing an HR <0.88 for PFS, which maintains at least 50% of the treatment effect observed in SOPHIA (HR =0.76) (12). Approximately 70 PFS events would be needed to ensure at least 73% power of demonstrating consistency. A target sample size of 120 was then derived based on the following assumptions: an mPFS of 5 months in the control arm, and a 5% dropout rate. PFS by BICR and OS were assessed in the intention-to-treat (ITT) population, defined as all randomized patients. Missing data were censored. Kaplan-Meier methods were used to estimate the mPFS and median OS (mOS). The Brookmeyer-Crowley method was used to calculate the 95% CI for each median time to event. The stratified log-rank test was used to compare PFS and OS between the two arms, and a stratified Cox proportional hazards model was used to estimate the HRs and the 95% CIs. ORR and CBR were calculated in the response evaluable population (defined as all patients who are randomized and have measurable lesion at baseline), with the 95% CIs estimated by the Clopper-Pearson method. Safety analysis was done in the safety analysis set, defined as all patients who received at least one dose of study drug. Exploratory subgroup analyses on PFS were conducted based on age, ECOG score, number of metastatic sites, chemotherapy choice, disease stage at initial breast cancer diagnosis, prior lines of systemic therapy, treatment history of pyrotinib or lapatinib, treatment history of pertuzumab, hormone receptor status, HER2 status, and CD16A and CD32A genotypes (assessed using polymerase chain reaction amplification of blood DNA and DNA sequencing). All statistical analyses were performed using SAS (version 9.4).
Results
Study population
From February 4, 2020 to February 23, 2021, a total of 173 patients were screened and 123 Chinese patients (107 in Chinese mainland, 8 in Hong Kong, 8 in Taiwan) were enrolled at 33 centers and randomized to receive either margetuximab plus chemotherapy (n=62) or trastuzumab plus chemotherapy (n=61) (Figure 1). Overall, 99.2% of all patients were female, and the median age was 53 [31–73] years. Demographic and baseline characteristics in the ITT population are summarized in Table 1. In total, 44.7% and 54.5% of patients had ECOG performance status of 0 and 1, respectively. All patients had metastatic disease. The most common sites of metastases included lymph node (64.2%), bone (49.6%), lung (49.6), and liver (40.7%). Among all, 52.8% were positive for combined hormone receptor status, and 90.2% were F carriers (F/F or F/V) for the Fcγ receptor CD16A gene. At baseline, all patients were pretreated with trastuzumab and 83.7%, 25.2%, and 11.4% of patients, respectively, were pretreated with TKIs, pertuzumab, and T-DM1. Patient demographic and baseline characteristics were in general well balanced between the two treatment groups. A greater proportion of patients (19.4%) in the margetuximab arm had brain metastasis than in the trastuzumab arm (9.8%).
Table 1
| Characteristics | Margetuximab plus chemotherapy (n=62) | Trastuzumab plus chemotherapy (n=61)† |
|---|---|---|
| Female, n (%) | 62 (100.0) | 60 (98.4) |
| Age (years), median (range) | 53.9 (32.6–67.0) | 52.9 (31.5–73.5) |
| ECOG performance status, n (%) | ||
| 0 | 27 (43.5) | 28 (45.9) |
| 1 | 35 (56.5) | 32 (52.5) |
| Missing | 0 | 1 (1.6) |
| Disease extent at screening, n (%) | ||
| Metastatic | 62 (100.0) | 60 (98.4) |
| Unknown | 0 | 1 (1.6) |
| Common sites of metastases (>10% of patients in either arm) at screening, n (%) | ||
| Lymph node | 37 (59.7) | 42 (68.9) |
| Bone | 32 (51.6) | 29 (47.5) |
| Lung | 30 (48.4) | 31 (50.8) |
| Liver | 24 (38.7) | 26 (42.6) |
| Brain | 12 (19.4) | 6 (9.8) |
| Skin | 7 (11.3) | 7 (11.5) |
| Pleural | 6 (9.7) | 8 (13.1) |
| Breast | 5 (8.1) | 7 (11.5) |
| HER2 expression, n (%) | ||
| ISH amplified | 16 (25.8) | 12 (19.7) |
| IHC 3+ | 46 (74.2) | 48 (78.7) |
| Unknown | 0 | 1 (1.6) |
| Combined ER and PgR status, n (%) | ||
| ER-positive, PgR-positive, or both | 33 (53.2) | 32 (52.5) |
| ER-negative and PgR-negative | 29 (46.8) | 28 (45.9) |
| Unknown | 0 | 1 (1.6) |
| Fcγ receptor CD16A genotype, n (%) | ||
| F/F or F/V | 56 (90.3) | 55 (90.2) |
| F/F | 33 (53.2) | 28 (45.9) |
| F/V | 23 (37.1) | 27 (44.3) |
| V/V | 4 (6.5) | 5 (8.2) |
| Unknown | 2 (3.2) | 1 (1.6) |
| No. of prior lines of systemic therapy‡, n (%) | ||
| ≤2 | 29 (46.8) | 28 (45.9) |
| >2 | 33 (53.2) | 32 (52.5) |
| Unknown | 0 | 1 (1.6) |
| No. of prior lines of anti-HER2 therapy, n (%) | ||
| ≤2 | 34 (54.8) | 32 (52.5) |
| >2 | 28 (45.1) | 28 (45.9) |
| Unknown | 0 | 1 (1.6) |
| Prior anti-HER2 therapy, n (%) | ||
| Trastuzumab | 62 (100.0) | 60 (98.4)† |
| Pyrotinib | 48 (77.4) | 41 (67.2) |
| Lapatinib | 16 (25.8) | 15 (24.6) |
| Pertuzumab | 13 (21.0) | 18 (29.5) |
| T-DM1 | 5 (8.1) | 9 (14.8) |
†, one patient randomized to the trastuzumab arm was found to meet an exclusion criterion (having leptomeningeal metastases or ongoing, symptomatic brain metastases) after randomization and was excluded accordingly without receiving any study treatment; for this patient, only the age, sex, race, and ethnicity had been entered into the study database while the other baseline characteristics were unavailable (shown as missing or unknown in Table 1); ‡, counted adjuvant therapy as one line of therapy if progressed within 6 months. ITT, intention-to-treat; ECOG, Eastern Cooperative Oncology Group; HER2, human epidermal growth factor receptor 2; ISH, in situ hybridization; IHC, immunohistochemical; ER, estrogen receptor; PgR, progesterone receptor; T-DM1, ado-trastuzumab emtansine.
Efficacy
By the time of data cut-off (September 3, 2021), there were 33 (53.2%) BICR-assessed PFS events (no death) in the margetuximab arm and 37 (60.7%) PFS events (including one death) in the trastuzumab arm. Compared with trastuzumab plus chemotherapy, margetuximab plus chemotherapy prolonged the BICR-assess PFS [mPFS: 5.52 (95% CI: 4.14–8.34) vs. 4.14 (95% CI: 2.76–5.52) months; HR =0.69; 95% CI: 0.43–1.12; P=0.128) (Figure 2). Consistently, margetuximab plus chemotherapy also prolonged the investigator-assessed PFS [mPFS: 5.52 (95% CI: 4.14–7.85) vs. 4.04 (95% CI: 1.71–5.06) months; HR =0.63; 95% CI: 0.41–0.96; P=0.032). Nine and 11 deaths occurred, respectively, in the margetuximab and trastuzumab arms, and the mOS had not been reached (Figure 3). BICR-assessed best overall response are summarized in Table 2. The tumor response results are summarized in Table 3. ORR was 25.5% (14/55, 95% CI: 14.67–39.00%) in the margetuximab arm and 12.5% (7/56, 95% CI: 5.18–24.07%) in the trastuzumab arm (P=0.083). CBR was 32.7% (18/55, 95% CI: 20.68–46.71%) in the margetuximab group and 14.3% (8/56, 95% CI: 6.38–26.22%) in the trastuzumab arm (P=0.025). The median DoR was not yet reached in the margetuximab arm (95% CI: 4.14 months–not evaluable), and was 7.0 (95% CI: 2.33–7.20) months in the trastuzumab arm. For the prespecified subgroup analyses, margetuximab plus chemotherapy showed a trend towards greater benefit in prolonging PFS than trastuzumab plus chemotherapy in most of the subgroups (Figure 4).
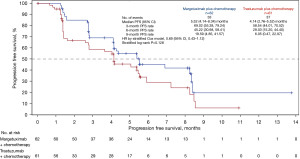
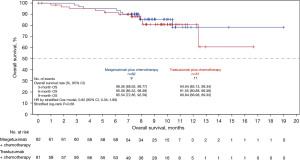
Table 2
| Responses | Margetuximab plus chemotherapy (n=55) | Trastuzumab plus chemotherapy (n=56) |
|---|---|---|
| Overall best response, n (%) | ||
| Confirmed CR | 0 | 1 (1.8) |
| Confirmed PR | 14 (25.5) | 6 (10.7) |
| SD | 33 (60.0) | 29 (51.8) |
| PD | 6 (10.9) | 17 (30.4) |
| NE | 2 (3.6) | 3 (5.4) |
| ORR, n (%) | 14 (25.5) | 7 (12.5) |
| 95% CI | 14.67, 39.00 | 5.18, 24.07 |
| P value | 0.083 | |
| CBR, n (%) | 18 (32.7) | 8 (14.3) |
| 95% CI | 20.68, 46.71 | 6.38, 26.22 |
| P value | 0.025 | |
| DoR (months), median | NR | 7.00 |
| 95% CI | 4.14, NE | 2.33, 7.20 |
BICR, blinded independent central review; CR, complete response; PR, partial response; SD, stable disease; PD, disease progression; NE, not evaluable; ORR, objective response rate; CI, confidence interval; CBR, clinical benefit rate; DoR, duration of remission; NR, not reached.
Table 3
| TEAEs | Margetuximab plus chemotherapy (n=62) | Trastuzumab plus chemotherapy (n=60) | |||
|---|---|---|---|---|---|
| All grade† | Grade ≥3‡ | All grade† | Grade ≥3‡ | ||
| Nonhematologic, n (%) | |||||
| AST increased | 17 (27.4) | 0 | 22 (36.7) | 2 (3.3) | |
| ALT increased | 17 (27.4) | 0 | 14 (23.3) | 0 | |
| Nausea | 14 (22.6) | 0 | 8 (13.3) | 0 | |
| Hyperglycemia | 12 (19.4) | 1 (1.6) | 6 (10.0) | 0 | |
| Urinary tract infection | 11 (17.7) | 0 | 10 (16.7) | 2 (3.3) | |
| Fatigue | 9 (14.5) | 0 | 9 (15.0) | 1 (1.7) | |
| Hypoalbuminemia | 9 (14.5) | 0 | 7 (11.7) | 0 | |
| Hypokalemia | 9 (14.5) | 1 (1.6) | 3 (5.0) | 1 (1.7) | |
| IRR | 9 (14.5) | 1 (1.6) | 1 (1.7) | 0 | |
| Diarrhea | 8 (12.9) | 0 | 9 (15.0) | 0 | |
| Constipation | 8 (12.9) | 0 | 9 (15.0) | 0 | |
| Vomiting | 8 (12.9) | 1 (1.6) | 9 (15.0) | 0 | |
| Pyrexia | 8 (12.9) | 1 (1.6) | 6 (10.0) | 0 | |
| Weight decreased | 8 (12.9) | 1 (1.6) | 6 (10.0) | 0 | |
| GGT increased | 8 (12.9) | 1 (1.6) | 10 (16.7) | 2 (3.3) | |
| Bone pain | 7 (11.3) | 0 | 2 (3.3) | 0 | |
| Hyponatremia | 4 (6.5) | 2 (3.2) | 5 (8.3) | 0 | |
| Skin rash | 3 (4.8) | 0 | 6 (10.0) | 0 | |
| Hematologic, n (%) | |||||
| Anemia | 42 (67.7) | 6 (9.7) | 34 (56.7) | 6 (10.0) | |
| White blood cell count decreased | 36 (58.1) | 10 (16.1) | 36 (60.0) | 13 (21.7) | |
| Neutrophil count decreased§ | 39 (62.9) | 13 (21.0) | 31 (50.8) | 12 (20.0) | |
| Platelets count decreased | 10 (16.1) | 0 | 7 (11.7) | 1 (1.7) | |
| Lymphocyte count decreased | 7 (11.3) | 2 (3.2) | 5 (8.3) | 3 (5.0) | |
| Febrile neutropenia | 2 (3.2) | 2 (3.2) | 1 (1.7) | 1 (1.7) | |
†, any-grade TEAE with ≥10% incidence in either treatment arm; ‡, grade ≥3 TEAE with an incidence of 2% in either treatment arm; §, combined with neutropenia. TEAE, treatment-emergent adverse event; AST, aspartate aminotransferase; ALT, alanine aminotransferase; IRR, infusion-related reaction; GGT, gamma-glutamyl transferase.

Safety
In the margetuximab and trastuzumab arms, respectively, TEAEs occurred in 98.4% (61/62) and 98.3% (59/60) of patients, and grade ≥3 TEAEs occurred in 45.2% (28/62) and 41.7% (25/60) of patients. Margetuximab or trastuzumab related TEAEs occurred in 75.8% (47/62) and 60.0% (36/60) of patients in the respective arms, and the proportions of patients with grade ≥3 study drug (margetuximab or trastuzumab) related TEAEs were 21.0% (13/62) and 15.0% (9/60), respectively. TEAEs leading to study drug discontinuation occurred in 16.1% (10/62) and 10.0% (6/60) of patients in the margetuximab and trastuzumab arms, respectively. No death due to TEAE was observed.
Common TEAEs (any-grade TEAE with ≥10% incidence or grade ≥3 TEAE with ≥2% incidence in either treatment arm) are summarized in Table 3. The most common (≥20% incidence) TEAEs in the margetuximab arm included anemia, decreased white blood cell count, decreased neutrophil count, increased aspartate aminotransferase, increased alanine transaminase, and nausea. Study drug-related IRR was more common in the margetuximab arm [12.9% (8/62)] than in the trastuzumab arm [1.7% (1/61)]. Most IRRs were of grades 1–2 (Table 3). IRR meeting the definition of AEs of special interest (i.e., grade ≥3 IRRs including cytokine release syndrome) was observed only in one patient, who was from the margetuximab arm and recovered on the same day after symptomatic treatment. All margetuximab-related IRRs occurred during the first administration of the drug, while the one IRR in the trastuzumab arm occurred in treatment cycle 5. All IRRs resolved with appropriate treatment. There was no dose delay or discontinuation of the study drug due to cardiac toxicity (such as a ≥15% decrease in LVEF as compared with baseline) in either treatment arm.
Discussion
This randomized, open-label, multicenter, phase II, bridging study evaluated the efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive MBC. Overall, the efficacy results demonstrated consistency with the clinical benefit of margetuximab observed in the global SOPHIA study. Margetuximab plus chemotherapy prolonged BICR-assessed PFS to a greater extent than trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive MBC, with a 31% relative risk reduction (HR =0.69, 95% CI: 0.43–1.12), which met the pre-defined consistency criterion for bridging success (HR <0.88). Consistently, a similar risk reduction was observed for investigator-assessed PFS (HR =0.63, 95% CI: 0.41–0.96). Considering that the OS data were immature, no conclusion can be drawn at this time about OS. In terms of tumor response, both ORR and CBR were greater in the margetuximab arm than in the trastuzumab arm [ORR: 25.5% (95% CI: 14.67–39.00%) vs. 12.5% (95% CI: 5.18–24.07%), P=0.083; CBR: 32.7% (95% CI: 20.68–46.71%) vs. 14.3% (95% CI: 6.38–26.22%), P=0.025], which were in line with those from SOPHIA [ORR: 22% (95% CI: 17.11–27.16%) vs. 16% (95% CI: 11.59–21.47%), P=0.06; CBR: 37% (95% CI: 30.81–42.48%) vs. 25% (95% CI: 19.58–30.04%), P=0.003]. Overall, the efficacy results indicated that the clinical benefit of margetuximab in Chinese patients is consistent with that observed in the global population in SOPHIA.
The subgroup analyses of BICR-assessed PFS (Figure 4) revealed several noteworthy observations. Firstly, patients pretreated with pyrotinib or lapatinib seemed to benefit more from margetuximab treatment than those who were not (HR =0.58, 95% CI: 0.34–0.98 vs. HR =0.82, 95% CI: 0.22–3.10), suggesting that margetuximab may be a suitable treatment option for patients pre-treated with TKIs. This is of high clinical relevance because patients pre-treated with TKIs represent an important population of interest among HER2-positive MBC patients in China. TKIs such as pyrotinib and lapatinib are recommended second-line treatments for Chinese HER2-positive MBC patients after trastuzumab treatment (4,6) and are widely adopted (more than 80% of patients in this study have been pre-treated with TKIs). Yet, patients may still develop therapeutic resistance to these treatments, and subsequent treatment choice remains controversial (10). Considering the treatment needs for these patients, the Chinese Society of Clinical Oncology has newly added a TKI-resistant patient stratification in the recently published Breast Cancer Guidelines 2022, but with only level II recommendations (high evidence level, but lower expert consensus and poor availability in China) (16). Data from the current study suggest that margetuximab may offer another treatment option for TKI-resistant patients in the local clinical setting.
Secondly, in terms of metastasis sites, patients with visceral metastasis appeared to benefit more from margetuximab as compared with those without (HR =0.59, 95% CI: 0.35–1.00 vs. HR =0.82, 95% CI: 0.26–2.57); and consistently, patients with liver metastasis also seemed to benefit more than those without (HR =0.53, 95% CI: 0.27–1.06 vs. HR =0.86, 95% CI: 0.43–1.71) (Figure 4). In particular, among patients with liver metastasis, margetuximab plus chemotherapy resulted in numerically much longer BICR-assessed PFS as compared with trastuzumab plus chemotherapy (5.39 vs. 2.76 months). However, the sample size of this study was relatively small. Additionally, results on the impact of visceral involvement on the efficacy of other anti-HER2 treatments have also been limited (17-19). Taken together, while these data suggest that margetuximab may help improve the clinical outcome of patients with visceral metastasis, especially that at the liver, further studies are required to better assess the impact of metastasis localization on treatment responses.
Thirdly, patients with the F/F or F/V alleles for CD16A experienced a risk reduction with margetuximab relative to trastuzumab (HR =0.68, 95% CI: 0.41–1.11), whereas patients who were not F carriers exhibited an opposite risk reduction trend (HR =1.15, 95% CI: 0.07–18.59). Similar findings were also reported in the SOPHIA trial (12). This is expected as the F variant is known to have a lower binding affinity to trastuzumab than the V variant, and margetuximab was specifically engineered for increased binding affinity to the F variant (11,13) in order to improve the efficacy in F carriers. Importantly, gene polymorphism data from SOPHIA, this study, as well as other independent studies, indicate that the vast majority (85–90%) of people (including both breast cancer patients and healthy individuals) are in fact F carriers (12,20,21), who would benefit more from margetuximab than from trastuzumab. Nevertheless, it should be noted that due to the small sample sizes in this study, these subgroup analyses are exploratory, and further research is needed to verify their clinical significance.
The safety results of this study were largely consistent with those from the SOPHIA study. The incidence of TEAE was high (~98%) but mostly comparable between the two treatment arms. In both studies, the most prominent between-treatment difference was the higher incidence of IRR in the margetuximab arm, but the incidence of grade ≥3 IRR was low (<2% in the margetuximab arm in both studies). In addition, margetuximab did not result in increased cardiac toxicity, another TEAE of special interest, compared with trastuzumab in either this study or the SOPHIA study. Some differences in hematologic TEAEs did exist between this and the SOPHIA study. Compared with patients in the SOPHIA study, the Chinese patients had much higher rates of anemia (~60% vs. ~20%), decreased white blood cell count (~60% vs. ~10%), and decreased neutrophil count (~60% vs. ~40%). A few factors could have potentially contributed to these discrepancies. Firstly, compared with SOPHIA, more patients received gemcitabine and vinorelbine, and fewer patients received capecitabine in this study, and capecitabine has lower hematologic toxicity than gemcitabine or vinorelbine (22-24). Secondly, Asian patients may be at greater risk of hematologic TEAEs than other races in general (25). Regardless, the incidences of these TEAEs were in fact similar across the two treatment arms in both this and the SOPHIA studies, suggesting that margetuximab is not associated with a greater incidence of these TEAEs than trastuzumab. Taken together, these results suggested that similar to the global patient sample in SOPHIA, margetuximab also has acceptable safety in Chinese patients, and precautionary actions can be taken to monitor and manage hematologic TEAEs regardless of treatment option due to high overall occurrence.
Prior treatments of patients enrolled in this study were highly reflective of the current treatment options for HER2-positive MBC patients in China and thus differed from those in the SOPHIA study. In the SOPHIA study, all but one patient had received pertuzumab and around 90% of the patients had received T-DM1 for prior anti-HER2 therapy (12). In contrast, only 25.2% and 11.4% of patients in this Chinese study were pretreated with pertuzumab and T-DM1, respectively, while the most common pretreatment was pyrotinib (used by 72.4% of patients). This is consistent with the treatment landscape in China, where TKIs are common second-line treatments while T-DM1 was only approved for advanced breast cancer in June 2021 (26). As such, the current study targeted a patient population broadly similar to the SOPHIA study (i.e., patients pretreated with multiple lines of anti-HER2 therapies and with no standard recommended treatment option due to further disease progression) while also capturing the China-specific clinical characteristics of pretreated HER2-positive MBC patients, so as to better inform the value of margetuximab to these patients under the local clinical setting. Based on the efficacy and safety results of margetuximab in this sample of patients not pretreated with pertuzumab or T-DM1, and the observation that patients pretreated with pertuzumab and T-DM1 in SOPHIA could still benefit from margetuximab treatment, margetuximab would be a valuable addition to the arsenal of treatment against HER2-positive MBC in China regardless of future local uptake of pertuzumab and T-DM1.
The study had a few limitations. Firstly, although the sample size is sufficient to demonstrate the consistency of margetuximab’s clinical benefit in Chinese patients with that in the global SOPHIA study, the small sample size limited the study’s statistical power for many secondary and exploratory outcomes, including the subgroup analyses for PFS, such that they remain inconclusive. Secondly, although clinical efficacy and safety were adequately assessed, this study did not measure patient-reported quality of life. As improving quality of life is also a key objective of treatment for advanced breast cancer, incorporating measures such as health-related quality of life could help better inform the effect of margetuximab on the patients’ overall wellbeing. Finally, this study only recruited Chinese patients, so the study results may not be generalizable to the larger Asian HER2-positive MBC patient population.
Conclusions
Despite the development of anti-HER2 therapies, MBC remains largely incurable, and multiple lines of therapy are typically required due to disease progression. Consistent with the findings from the SOPHIA study, margetuximab also prolonged PFS and was well tolerated in pretreated HER2-positive MBC patients in China, supporting its use in this patient population.
Acknowledgments
The authors thank all the patients and their families, investigators, and study staff for participating in this study. The authors acknowledge Ruilin Chen (Costello Medical, Singapore, sponsored by Zai Lab) for medical writing and editorial assistance in preparing this manuscript for publication, based on authors’ input and direction.
Funding: This work was sponsored by Zai Lab (Shanghai) Co., Ltd.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-35/rc
Data Sharing Statement: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-35/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-35/coif). This work was sponsored by Zai Lab (Shanghai) Co., Ltd. ZJ serves as an Editor-in-Chief of Translational Breast Cancer Research. QZ, XW, HW, YY, TS, SW, and YP serve as unpaid editorial board members of Translational Breast Cancer Research from Mar 2022 to Feb 2024. LY and YX are employees of Zai Lab (Shanghai) Co., Ltd. and hold stock and stock options of Zai Lab (Shanghai) Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Guideline for Good Clinical Practice. The study protocol was approved by institutional review board/independent ethics committee at each participating site. Informed consent was obtained from all individual participants in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- He J, Chen WQ, Li N, et al. China guideline for the screening and early detection of female breast cancer (2021, Beijing). Zhonghua Zhong Liu Za Zhi 2021;43:357-82. [PubMed]
- Ma F, Wu J, Fu L, et al. Interpretation of specification for breast cancer screening, early diagnosis, and treatment management in Chinese women. Journal of the National Cancer Center 2021;1:97-100. [Crossref]
- Breast Cancer Expert Committee of China Anti-Cancer Association. Guidelines and Standards for the Diagnosis and Treatment of Breast Cancer by the China Anti-Cancer Association (2021 edition). China Oncology 2021;31:954-1040.
- Breast Cancer Expert Committee of National Cancer Quality Control Center. Cancer Drug Clinical Research Committee of China Anti-Cancer Association. Guidelines for clinical diagnosis and treatment of advanced breast cancer in China (2020 Edition). Zhonghua Zhong Liu Za Zhi 2020;42:781-97.
- Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021;32:1475-95. [Crossref] [PubMed]
- Breast Cancer Expert Committee of the Chinese Society of Clinical Oncology, Breast Cancer Expert Committee of China Anti-Cancer Association. Expert consensus on clinical diagnosis and treatment of human epidermal growth factor receptor 2 positive breast cancer (2021 edition). National Medical Journal of China 2021;101:1226-31.
- Cesca MG, Vian L, Cristóvão-Ferreira S, et al. HER2-positive advanced breast cancer treatment in 2020. Cancer Treat Rev 2020;88:102033. [Crossref] [PubMed]
- Pondé N, Brandão M, El-Hachem G, et al. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev 2018;67:10-20. [Crossref] [PubMed]
- Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer 2020;126:4278-88. [Crossref] [PubMed]
- Hua Y, Li W, Jin N, et al. Treatment with pyrotinib-based therapy in lapatinib-resistant HER2-positive metastatic breast cancer: a multicenter real-world study. Ther Adv Med Oncol 2022;14:17588359221085232. [Crossref] [PubMed]
- Tarantino P, Morganti S, Uliano J, et al. Margetuximab for the treatment of HER2-positive metastatic breast cancer. Expert Opin Biol Ther 2021;21:127-33. [Crossref] [PubMed]
- Rugo HS, Im SA, Cardoso F, et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021;7:573-84. [Crossref] [PubMed]
- Nordstrom JL, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res 2011;13:R123. [Crossref] [PubMed]
- U.S. Food & Drug Administration. FDA approves margetuximab for metastatic HER2-positive breast cancer. 2021. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-margetuximab-metastatic-her2-positive-breast-cancer (Accessed Dec 10, 2021).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer (Version 1.2022). 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed Dec 10, 2021).
- Jiang Z, Li J, Chen J, et al. Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guidelines 2022. Transl Breast Cancer Res 2022;3:13. [Crossref]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Saura C, Oliveira M, Feng YH, et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 2020;38:3138-49. [Crossref] [PubMed]
- Dai M, Zhou Z, Wang X, et al. Association of FcγRIIIa-158V/F with systemic lupus erythematosus in a Chinese population. Int J Rheum Dis 2013;16:685-91. [Crossref] [PubMed]
- Litjens N, Peeters A, Gestel JK, et al. The FCGR3A 158 V/V-genotype is associated with decreased survival of renal allografts with chronic active antibody-mediated rejection. Sci Rep 2021;11:7903. [Crossref] [PubMed]
- Capecitabine (Xeloda) [package insert]. San Francisco: Genetech USA, Inc.; 2021.
- Gemcitabine (Gemzar) [package insert]. Indianapolis: Lilly USA, LLC; 2019.
- Vinorelbine (Navelbine) [package insert]. Parsippany: Pierre Fabre Pharmaceuticals, Inc.; 2020.
- Ettl J, Im SA, Ro J, Masuda N, et al. Hematologic adverse events following palbociclib dose reduction in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: pooled analysis from randomized phase 2 and 3 studies. Breast Cancer Res 2020;22:27. [Crossref] [PubMed]
- Kadcyla® Trastuzumab Emtansine for Injection Prescribing Information. Shanghai: Roche Pharmaceuticals (Shanghai) Ltd.; 2021.
Cite this article as: Zhang Q, Ouyang Q, Li W, Chiu J, Yan M, Lu YS, Sun S, Li H, Du Y, Wang X, Sun T, Yin Y, Wang H, Ye F, Shen K, Wang J, Pan Y, Wang S, Yang J, Wu X, Dai MS, Cheng J, Teng Y, Su F, Wu X, He J, Fu P, Yang L, Xin Y, Wang X, Jiang Z. Efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive advanced metastatic breast cancer: results from a randomized, open-label, multicenter, phase II bridging study. Transl Breast Cancer Res 2022;3:31.



