
Molecular subtypes predict the prognosis of male breast cancer: a retrospective cohort study
Introduction
According to the National Institutes of Health (NIH)’s data, breast cancer has become the most common cancer in the United States (1). Rather than female breast cancer, male breast cancer accounts for small proportion of all the breast cancer, about 0.5–1% (2,3). Due to the rarity, the knowledge about male breast cancer is lacking. At present, the main understanding of male breast cancer is that it occurs more often in men with diseases leading to high estrogen status, such as Klinefelter’s syndrome characterized by rare 47, XXY chromosomal abnormalities and significant hormonal changes (4,5); gynecomastia (6), an estrogen excesses disease, such as exogenous estrogen intake (7); anti-androgen therapy (8); liver diseases (9,10); orchitis/epididymitis and obesity (5). Like female breast cancer, most male breast cancer is in estrogen receptor (ER) positive status, and that happens more often than that in female ones (11). ER positive rates were reported at about 90–92%, while the progesterone receptor (PR) positive rates were 81–96% around the world (12-14). In contrast to the HR status, the rate of human epidermal growth factor receptor 2 (HER2) overexpression is lower in male breast cancer than in female breast cancer, occurring in about 2–15% of the male breast cancer patients (15).
Molecular subtype mainly composed with hormone receptor (HR) status and HER2 status. Since first revealed in 2000, it has been widely recognized as an intrinsic subtype and helped to predict the features and prognosis of female breast cancer. In recent years, many studies have showed that male breast cancer is not equally to female ones. And there are very few studies concerned about the molecular subtype of male breast cancer, and all these studies are based on single institution or small cohorts. According to previous studies, HR+/HER2− subtype accounts for the majority of male breast cancer, but other molecular subtypes are rarely concerned. Meanwhile, whether molecular subtype of male breast cancer has the same effect as female one’s remains unclear (12-17). Since 2010, the Surveillance, Epidemiology, and End Results (SEER) database recorded molecular subtype of breast cancer, therefore, we conducted a population-based study to investigate the association between different molecular subtypes in male breast cancer and its characteristics and outcomes. We present the following article in accordance with the STROBE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-24/rc).
Methods
Database and patients selection
It is a retrospective cohort study. All the data were collected from the National Cancer Institute’s SEER program, which contains cancer incidence and survival data from population-based central cancer registries covering approximately 28% of the population in United States. The SEER database provides data on patients’ demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, follow-up status and some other information. SEER database started to collect HER2 status at the beginning of 2010. Thus, year of 2010 was selected as the starting point in this study. We conducted the data collection in 2020 and current study was designed to set the follow-up time as 5 years. Therefore, the data from SEER 18 Regs Research Data + Hurricane Katrina impacted Louisiana Cases, Nov 2016 sub [2000–2014] “Katrina/Rita Population Adjustment” were chosen in current study and all the data were restricted to this cohort to avoid potential bias in base populations. The data in SEER database were entered by trained registrars who collected dates and causes of death of each case’s death certificates from US Census Bureau.
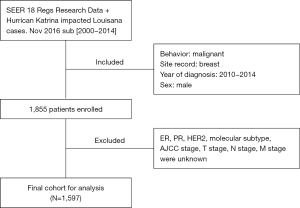
Stepwise cohort ascertainment is shown in Figure 1. Year of diagnosis between 2010 and 2014, a total of 1,855 patients diagnosed as male breast cancer was extracted, histologic types were identified to use the code defined by ICD-O-3 (International Classification of Diseases for Oncology third edition): duct carcinoma =8,500, lobular carcinoma =8,520. Information of age at diagnosis, race (White, Black, Asian), Contract Health Service Delivery Areas (CHSDA) Region (East, Northern plains, Pacific coast, Southwest, Alaska), grade (well, moderately, poorly, undifferentiated, unknown), laterality (left, right, bilateral, unknown), tumor size, American Joint Committee on Cancer (AJCC) stage (stage I, stage II, stage III, stage IV, unknown) (18), T stage (T0, T1, T2, T3, T4, unknown), N stage (N0, N1, N2, N3, unknown), M stage (M0, M1, Unknown), ER status (positive, negative, borderline, unknown), PR status (positive, negative, borderline, unknown), HER2 status (positive, negative, unknown), histologic types (duct carcinoma, lobular carcinoma, others, unknown), surgery (breast-conserving surgery, mastectomy, unperformed) and follow-up status (alive, dead) were extracted. Then, those patients with AJCC stage unknown, T stage unknown, N stage unknown, M stage unknown, subtype unknown, surgery did not perform were excluded. Finally, the cohort of 1,597 cases were enrolled in this study.
ER and PR status was determined by immunohistochemical staining. ER or PR borderline was treated as positive in current study. HER2 status was determined by immunohistochemical staining combined with FISH test if necessary. HR positive was defined as ER positive with or without PR positive, molecular subtypes were expressed as follow: HR+/HER2−, HR+/HER+, HR−/HER2+ and triple negative (TN).
Follow-up time was lasted to 5 years (60 months). Due to the limitation of SEER database, the clinician information of each case, type of surgical approach, radiation dose, whether receiving chemotherapy, endocrine therapy or immunotherapy was unknown. SEER database only records each case’s date of death and cause of death, but the situation of tumor recurrence. Outcomes in current study were expressed as 5-year overall survival rate (5y-OS) and 5-year cause-specific survival rate (5y-CSS) and death risk (hazard ratio). 5y-OS represented the cases alive at the end of the study or alive at their last follow-up. 5y-CSS represented the cases alive at the end of the study or died of other causes except breast cancer. Follow-up time was calculated for each case by contracting date from “Completed Months of Follow-Up” option.
Statistical analysis
Demographic and clinicopathological characteristics were compared using the Pearson chi-square test for categorical variables, medians were used as central parameters, 25% and 75% quartiles were used as dispersion parameters. 5y-OS and 5y-CSS were investigated by Kaplan-Meier analyses. CSS was defined as the death due to breast cancer. Survival curves were calculated and Log-rank test were performed. Multivariable Cox proportional hazards regression model analysis was performed to identify the independent predictors for poor survival with potential risk factors, including ER, PR, HER2, age, molecular subtype, tumor size, AJCC stage, Grade, TNM stage and race, after controlling other factors. Analyses were conducted using SPSS version 21.0 (IBM Corp, Armonk, NY, USA). A two-sided P<0.05 were considered statistically significant, and 95% confidence intervals (CI) were used to indicate confidence level. In current study, data analysis was performed by two authors seperately to avoid statistical bias. We compared two ones’ results in every section. If results were inconsistent, we would seek for the assistance of statistical experts.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine (No. 2017KY117) and informed consent was taken from all the patients.
Results
Demographic and clinicopathological characteristics
According to our criteria, 1,855 male breast cancer patients met the criteria during 2010–2014 totally. Then those patients with ER, PR, HER2, molecular subtype, AJCC stage and T, N, M stage displayed as unknown were excluded. Finally, a total of 1,597 cases were enrolled in current study, with median age being 66 years (26–98 years), average tumor size being 3.97 cm (0–8 cm), follow-up time being 46 months (1–60 months).
Patient demographics and clinicopathologic characteristics are summarized in Table 1. Patients with older age (>65 years) accounted for majority of the patients (54.7%). Moderated grade was the most common grade (50%); 97.1% of the patents were ER positive and 90.5% of the patients were PR positive. Patients with HER2 positive accounted for 12.2% of the total cases. There were 1,373 cases in the HR+/HER2− group (86%), 182 in the HR+/HER+ group (11.4%), 13 in the HR−/HER2+ group (0.8%) and 29 in the TN group (1.8%) respectively.
Table 1
| Variables | N | % |
|---|---|---|
| Age at diagnosis (26–98 years) | ||
| <35 | 8 | 0.5 |
| 35–49 | 175 | 11 |
| 50–65 | 540 | 33.8 |
| >65 | 874 | 54.7 |
| Tumor size (0–8 cm) | ||
| <0.5 cm | 57 | 3.6 |
| 0.5–1 cm | 105 | 6.6 |
| 1–2 cm | 519 | 32.5 |
| 2–5 cm | 715 | 44.8 |
| >5 cm or T4 | 201 | 12.6 |
| Race | ||
| White | 1,270 | 79.5 |
| Black | 243 | 15.2 |
| Asian | 84 | 5.3 |
| CHSDA region | ||
| East | 701 | 43.9 |
| Northern plains | 154 | 9.6 |
| Pacific coast | 685 | 42.9 |
| Southwest | 56 | 3.5 |
| Alaska | 1 | 0.1 |
| Grade | ||
| Well | 187 | 11.7 |
| Moderately | 799 | 50 |
| Poorly | 538 | 33.7 |
| Undifferentiated | 4 | 0.3 |
| Unknown | 69 | 4.3 |
| Laterality | ||
| Left | 855 | 53.5 |
| Right | 739 | 46.3 |
| Bilateral | 0 | 0 |
| Unknown | 3 | 0.2 |
| AJCC stage | ||
| Stage I | 525 | 32.9 |
| Stage II | 686 | 43 |
| Stage III | 265 | 16.6 |
| Stage IV | 121 | 7.6 |
| T stage | ||
| T0 | 7 | 0.4 |
| T1 | 674 | 42.2 |
| T2 | 715 | 44.8 |
| T3 | 60 | 3.8 |
| T4 | 141 | 8.8 |
| N stage | ||
| N0 | 888 | 55.6 |
| N1 | 485 | 30.4 |
| N2 | 142 | 8.9 |
| N3 | 82 | 5.1 |
| M stage | ||
| M0 | 1,476 | 92.4 |
| M1 | 121 | 7.6 |
| ER status | ||
| Positive | 1,550 | 97.1 |
| Negative | 46 | 2.9 |
| Borderline | 1 | 0.1 |
| PR status | ||
| Positive | 1,446 | 90.5 |
| Negative | 148 | 9.3 |
| Borderline | 3 | 0.2 |
| HER2 status | ||
| Positive | 195 | 12.2 |
| Negative | 1,402 | 87.8 |
| Molecular subtypes | ||
| HR+/HER2− | 1,373 | 86 |
| HR+/HER2+ | 182 | 11.4 |
| HR−/HER2+ | 13 | 0.8 |
| Triple-negative | 29 | 1.8 |
| Histologic types | ||
| Duct carcinoma | 1,321 | 82.7 |
| Lobular carcinoma | 12 | 0.8 |
| Others | 264 | 16.5 |
CHSDA, Contract Health Service Delivery Areas; AJCC, American Joint Committee on Cancer; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
Demographics and clinicopathologic characteristics within different molecular subtypes were showed in Table 2. There were significant differences in distributions in age, race, grade, tumor size and AJCC stage between the molecular subtypes. As presented in Table 2, older patients (>65 years) distributed mostly in TN group. Moreover, more Black patients happened to be HER2+/HR+ subtype than other races while more Asian patients within the HER2+/HR− subtype. Furthermore, poorly grade mainly distributed in TN subtype or HER2+/HR− subtype and moderated grade mainly happened in HER2−/HR+ subtype. In addition, there were more TN patients in higher AJCC stage, however, as to tumor size, there were more percentage of the HER2+/HR+ patients in large size (>5 cm or T4).
Table 2
| Variables | HER2+/HR+, N=182 (%) | HER2+/HR−, N=13 (%) | HER2−/HR+, N=1,373 (%) | TN, N=29 (%) | P value |
|---|---|---|---|---|---|
| Age at diagnosis, years | 0.001 | ||||
| <35 | 1 (0.5) | 1 (7.7) | 6 (0.4) | 0 (0) | |
| 35–49 | 24 (13.2) | 3 (23.1) | 145 (10.6) | 3 (10.3) | |
| 50–65 | 78 (42.9) | 3 (23.1) | 450 (32.8) | 9 (31.0) | |
| >65 | 79 (43.4) | 6 (46.2) | 772 (56.2) | 17 (58.6) | |
| Race | 0.02 | ||||
| White | 137 (75.3) | 9 (69.2) | 1,105 (80.5) | 19 (65.5) | |
| Black | 37 (20.3) | 1 (7.7) | 198 (14.4) | 7 (24.1) | |
| Asian | 8 (4.4) | 3 (23.1) | 70 (5.1) | 3 (10.3) | |
| Grade | <0.001 | ||||
| Well | 4 (2.2) | 1 (7.7) | 182 (13.3) | 0 (0) | |
| Moderately | 77 (42.3) | 1 (7.7) | 718 (52.3) | 3 (10.3) | |
| Poorly | 91 (50.0) | 8 (61.5) | 417 (30.4) | 22 (75.9) | |
| Undifferentiated | 0 (0) | 0 (0) | 2 (0.1) | 2 (6.9) | |
| Unknown | 10 (5.5) | 3 (23.1) | 54 (3.9) | 2 (6.9) | |
| AJCC stage | <0.001 | ||||
| Stage I | 38 (20.9) | 1 (7.7) | 480 (35.0) | 6 (20.7) | |
| Stage II | 84 (46.2) | 5 (38.5) | 589 (42.9) | 8 (27.7) | |
| Stage III | 35 (19.2) | 4 (30.8) | 221 (16.1) | 5 (17.2) | |
| Stage IV | 25 (13.7) | 3 (23.1) | 83 (6.0) | 10 (34.5) | |
| Histologic types | 0.12 | ||||
| Duct carcinoma | 159 (70.9) | 10 (76.9) | 1,133 (82.5) | 19 (65.5) | |
| Lobular carcinoma | 1 (0.5) | 0 (0) | 11 (0.8) | 0 (0) | |
| Others | 22 (12.1) | 3 (23.1) | 229 (16.7) | 10 (34.5) | |
| Tumor size | 0.001 | ||||
| <0.5 cm | 1 (0.5) | 0 (0) | 54 (3.9) | 2 (6.9) | |
| 0.5–1 cm | 4 (2.2) | 1 (7.7) | 99 (7.2) | 1 (3.4) | |
| 1–2 cm | 44 (24.2) | 2 (15.4) | 469 (34.2) | 4 (13.8) | |
| 2–5 cm | 97 (53.3) | 9 (69.2) | 590 (43.0) | 19 (65.5) | |
| >5 cm or T4 | 36 (19.8) | 1 (7.7) | 161 (11.7) | 3 (10.3) |
HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TN, triple negative; AJCC, American Joint Committee on Cancer.
5y-OS and 5y-CSS comparison
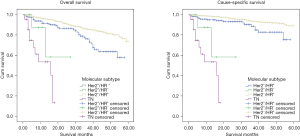
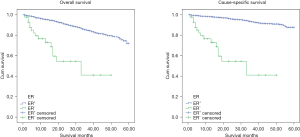
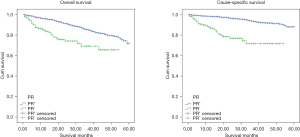
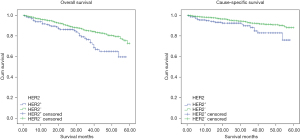
Kaplan-Meier analysis was carried out to determine 5y-OS and 5y-CSS. As showed in Figure 2, the molecular subtypes differed significantly in 5y-OS (P<0.001) and 5y-CSS (P<0.001). 5y-OS was 73% (HER2−/HR+), 56.1% (HER2+/HR+), 72.6% (HER2+/HR−), 43.2% (TN) and 5y-CSS rates was 89.2% (HER2−/HR+), 78.4% (HER2+/HR+), 72.6% (HER2+/HR−) and 43.2% (TN) separately. It establishes the survival sequence as HER2−/HR+ > HER2+/HR+ > HER2+/HR− > TN. In addition, 5y-OS and 5y-CSS was also compared based on ER, PR and HER2 status respectively, and the result showed significantly differences in all the comparisons (Figures 3-5). In Figure 6, 5y-OS and 5y-CSS of different races was presented, it is revealed that in the 5y-OS comparison, the sequence was Asian > White > Black, while in the 5y-CSS comparison, the sequence was White > Asian > Black.

Cox proportional hazards regression model analysis was performed as multivariate analysis, demonstrating that age ≥65 years (P=0.001, hazard ratio =2.136), ER negative (P=0.02, hazard ratio =2.481), PR negative (P=0.007, hazard ratio =2.294), TN subtype (P<0.001, hazard ratio =10.676), AJCC stage IV (P<0.001, hazard ratio =21.222), tumor size >5 cm or T4 (P<0.001, hazard ratio =2.577), Stage M1 (P=0.001, hazard ratio =4.519) and Black race (P=0.002, hazard ratio =2.322) were independent prognostic factors for poorer CSS (Table 3).
Table 3
| Variables | OS | CSS | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| ER | 0.01 | 0.02 | |||
| Positive | Reference | Reference | |||
| Negative | 2.639 (1.323, 5.263) | 2.481 (1.159, 5.319) | |||
| PR | 0.04 | 0.007 | |||
| Positive | Reference | Reference | |||
| Negative | 1.637 (1.031, 2.604) | 2.294 (1.256, 4.184) | |||
| HER2 | 0.14 | 0.67 | |||
| Negative | Reference | Reference | |||
| Positive | 1.333 (0.913, 1.946) | 1.126 (0.65, 1.948) | |||
| Molecular subtype | <0.001 | <0.001 | |||
| HER2+/HR+ | Reference | Reference | |||
| HER2+/HR− | 1.156 (0.268, 4.98) | 1.981 (0.4, 9.821) | |||
| HER2−/HR+ | 0.619 (0.423, 0.906) | 0.619 (0.355, 1.079) | |||
| TN | 5.731 (2.762, 11.893) | 10.676 (4.441, 25.665) | |||
| Tumor size | <0.001 | <0.001 | |||
| ≤0.5 cm | Reference | Reference | |||
| 0.5–1 cm | 0.715 (0.272, 1.876) | 0.53 (0.127, 2.212) | |||
| 1–2 cm | 0.544 (0.256, 1.158) | 0.408 (0.142, 1.179) | |||
| 2–5 cm | 1.307 (0.643, 2.655) | 1.114 (0.434, 2.86) | |||
| >5 cm or T4 | 2.727 (1.317, 5.646) | 2.577 (0.978, 6.792) | |||
| AJCC stage | <0.001 | <0.001 | |||
| Stage I | Reference | Reference | |||
| Stage II | 1.693 (1.108, 2.589) | 1.745 (0.818, 3.723) | |||
| Stage III | 2.636 (1.671, 4.159) | 3.003 (1.363, 6.617) | |||
| Stage IV | 8.799 (5.554, 13.941) | 21.222 (10.377, 43.4) | |||
| Grade | 0.68 | 0.43 | |||
| Well | Reference | Reference | |||
| Moderately | 1.399 (0.242, 8.087) | 1.772 (0.203, 15.444) | |||
| Poorly | 1.821 (0.343, 9.675) | 2.064 (0.291, 14.658) | |||
| Undifferentiated | 1.9 (0.361, 9.995) | 2.81 (0.397, 19.863) | |||
| T stage | <0.001 | 0.003 | |||
| T1 | Reference | Reference | |||
| T2 | 2.061 (1.442, 2.947) | 2.342 (1.331, 4.12) | |||
| T3 | 4.039 (2.229, 7.317) | 4.631 (1.933, 11.091) | |||
| T4 | 1.565 (0.799, 3.068) | 1.406 (0.525, 3.763) | |||
| N stage | 0.10 | 0.36 | |||
| N0 | Reference | Reference | |||
| N1 | 2.012 (1.04, 3.893) | 1.484 (0.905, 2.433) | |||
| N2 | 2.096 (1.131, 3.882) | 1.085 (0.499, 2.359) | |||
| N3 | 1.377 (0.705, 2.689) | 1.63 (0.776, 3.423) | |||
| M stage | <0.001 | 0.001 | |||
| M0 | Reference | Reference | |||
| M1 | 3.719 (1.99, 6.951) | 4.519 (1.929, 10.587) | |||
| Age at diagnosis | <0.001 | 0.001 | |||
| <65 years | Reference | Reference | |||
| ≥65 years | 2.181 (1.606, 2.96) | 2.136 (1.372, 3.324) | |||
| Race | 0.27 | 0.002 | |||
| White | Reference | Reference | |||
| Black | 1.281 (0.896, 1.832) | 2.322 (1.442, 3.74) | |||
| Asian | 0.749 (0.327, 1.715) | 1.673 (0.667, 4.197) | |||
OS, overall survival; CSS, cause-specific survival; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; TN, triple negative; AJCC, American Joint Committee on Cancer.
Discussion
Compared with female breast cancer, male breast cancer is a rare disease. Current understanding regarding male breast cancer was largely extrapolated from the female cancers. Male breast cancer is commonly found in elderly men, and the incidence rate increases with age, different with the bimodal type as female breast cancer. In current study, major group of age at diagnosis was the oldest group (age >65 years), accounting for 54.7% of the total cases. Only 11.5% patients were diagnosed under 50 years. As refer to molecular subtypes, TN group consisted the oldest patients (58.6% >65 years) and HER2+/HR+ group contained the youngest patients (43.4% >65 years).
Like female breast cancer, ductal carcinoma is the most common histologic types of male breast cancer, in our study it consisted 82.7% of the patients, however, dislike female, lobular carcinoma is not as much as in females, it only consisted 0.8% of all the patients, while other histologic types, just including medullary carcinoma, mucinous carcinoma, papillary carcinoma, tubular carcinoma and so on, consisted 16.5% of all the patients. These findings were consistent with previous study (19).
Grade was strongly association with tumor proliferation, in SEER database, grade 1–4 was expressed as well, moderately, poorly differentiated and undifferentiated. In general, moderately differentiated accounted for the most common grade, but when by molecular subtypes, only in HER2−/HR+ group, it ranked top grade, in other three subtypes, poorly differentiated is the most common grade type, which could partially explain the best outcome of HER2−/HR+ subtype. In our study, grade was not turned to be an independent prognostic factor, which was consistent with some previous reports (12,20).
Tumor stage is an important factor for treatment and prognosis, Fentiman et al. reported the percentage of male breast cancer in AJCC stage distributing as follow: 37% in stage I, 21% in stage II, 33% in stage III and 9% in stage IV (21). In current study, stage I accounting for 32.9%, stage II accounting for 43%, stage III accounting for 16.5% and stage IV accounting for 7.6%. The mainly differences were in stage II and stage III. In molecular subtype, besides TN group distributing most in stage IV, other groups were largely enrolled in stage II.
In our study, ER and PR positive rate was 97.1% and 90.5% respectively, higher than previous report about male breast cancer (14,19), and this rate was much higher than female too (22-24). HR positive was a favorable prognostic factor in our study, and 5y-OS and 5y-CSS of ER positive and PR positive group were better than negative groups.
Contrast to HR, HER2 expression was not as high as in female breast cancer. In our study, HER2 positive rate was 12.2%, while it is usually considered to be 20–25% in female breast cancer (25). Survival analysis was also performed to compare the subgroup classified by HER2 status and showed differences. However, in multivariate analysis, HER2 status was not recognized as a prognostic factor in male breast cancer. This result was inconsistent with several previous reports (26,27), but Leone et al.’s study showed same result with us (28).
Molecular subtype of breast cancer, first being suggested by Perou et al. in 2000 (29), classified breast cancer into four mainly types by using the expression of ER, PR and HER2. It was considered revealing the intrinsic features of the tumors and has already been used extensively in breast cancer therapeutic strategy making and prognosis predicting. In SEER database, Ki67 was not recorded, so molecular subtype was expressed as HR+/HER2−, HR+/HER+, HR−/HER2+ and TN. In this study, these subgroups differed significantly in 5y-OS and 5y-CSS, establishing the survival sequence as HER2−/HR+ > HER2+/HR+ > HER2+/HR− > TN, and this sequence was similar to female breast cancer.
There was rarely prospective study on male breast cancer systemic treatment and many of the strategies were extrapolated from females. Because 20–25% of estrogen in men is produced in testes independently of aromatase enzymes, aromatase inhibitors can hardly inhibit estrogen production singly (30). Tamoxifen became the standard endocrine treatment for male breast cancer and was shown to be effective in 90% of ER positive male breast cancer patients (31). Unfortunately, men often experience bothersome side effects from tamoxifen, such as hot flashes or sexual dysfunction, resulting 25% of the patients suspended treatment in early times (32). Moreover, not all ER positive male breast cancer have the same performance as female. Johansson et al.’s study revealed that some of the male patients might have an inactive ER pathway leading to no response to endocrine treatment in the same way as women do (20).
There are no prospective trials on trastuzumab therapy in HER2 overexpression male breast cancer patients too. In some studies, male breast cancer patients got good response to trastuzumab treatment so it is also recommended to male breast cancer (33).
At the end of this study, we compared 5y-OS and 5y-CSS between different races. Although both showed differences in univariate analysis, only 5y-CSS comparison showed significant in multivariate analysis, establishing the favorable prognosis as White > Asian > Black. But in univariate analysis of 5y-OS, the sequence was different, showing Asian > White > Black. The same thing was Black men showed worst outcomes in both 5y-OS and 5y-CSS, but White men and Asian men have different outcomes in overall survival and breast cancer specific survival. This may be explained by other disease occurred differently between white and Asian men, such as cardiovascular disease. In Chavez-Macgregor et al.’s study (34), they also found the sequence as Hispanic > Asian/Pacific islander > non-Hispanic Whites > Black in overall survival, and after adjusting for subtype, stage and age, also showed no significant differences by races. But their study did not compare the breast cancer specific survival. Different prognoses between races are associated with many factors as gene, insurance, income, education, and facility type (35).
Current study first revealed the molecular subtype characteristics based on a large population-based cohort and present some different result compared to previous reports. As far as the incidence of male breast cancer is concerned, current study is a large sample cohort study, and the result has relatively good external validity. However, most cases enrolled in current study were white men, more Asian men’s data are still needed to further explore the meaning for Asian people. Of course, we also acknowledged the limitations of current study. It was a retrospective study and lacked the data about recurrence. And treatment method was not included in our study due to the recording limitation of SEER database. Due to the rarity of male breast cancer, more information needs to be explored, and prospective international multi-institution study need be hold in future.
Conclusions
Male breast cancer appears to be diverse in prognosis by molecular subtypes as female breast cancer. It could be a predictor for prognosis and assistant male breast cancer treatment.
Acknowledgments
Funding: This study was supported by the Shanghai Songjiang District Science and Technology Research (Medical and Health) Project (grant number 20SJKJGG304) and Shanghai General Hospital Characteristic Research Project (grant number CTCCR-2021C19).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-24/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-24/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine (No. 2017KY117) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Stat Facts: Female Breast Cancer. National Cancer Institute. 2017. Available online: https://seer.cancer.gov/statfacts/html/breast.html. Based on SEER 18 2010-2014.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN--a Nordic tool for cancer information, planning, quality control and research. Acta Oncol 2010;49:725-36. [Crossref] [PubMed]
- Hultborn R, Hanson C, Köpf I, et al. Prevalence of Klinefelter's syndrome in male breast cancer patients. Anticancer Res 1997;17:4293-7.
- Brinton LA, Carreon JD, Gierach GL, et al. Etiologic factors for male breast cancer in the U.S. Veterans Affairs medical care system database. Breast Cancer Res Treat 2010;119:185-92. [Crossref] [PubMed]
- Guénel P, Cyr D, Sabroe S, et al. Alcohol drinking may increase risk of breast cancer in men: a European population-based case-control study. Cancer Causes Control 2004;15:571-80. [Crossref] [PubMed]
- Thomas DB, Jimenez LM, McTiernan A, et al. Breast cancer in men: risk factors with hormonal implications. Am J Epidemiol 1992;135:734-48. [Crossref] [PubMed]
- Di Lauro L, Barba M, Pizzuti L, et al. Androgen receptor and antiandrogen therapy in male breast cancer. Cancer Lett 2015;368:20-5. [Crossref] [PubMed]
- Lenfant-Pejovic MH, Mlika-Cabanne N, Bouchardy C, et al. Risk factors for male breast cancer: a Franco-Swiss case-control study. Int J Cancer 1990;45:661-5. [Crossref] [PubMed]
- Sørensen HT, Friis S, Olsen JH, et al. Risk of breast cancer in men with liver cirrhosis. Am J Gastroenterol 1998;93:231-3. [Crossref] [PubMed]
- Anderson WF, Jatoi I, Tse J, et al. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol 2010;28:232-9. [Crossref] [PubMed]
- Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men: a population-based study. Cancer 2004;101:51-7. [Crossref] [PubMed]
- Fentiman I. Male breast cancer: a review. Ecancermedicalscience 2009;3:140. [Crossref] [PubMed]
- Cutuli B, Le-Nir CC, Serin D, et al. Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit Rev Oncol Hematol 2010;73:246-54. [Crossref] [PubMed]
- Gómez-Raposo C, Zambrana Tévar F, Sereno Moyano M, et al. Male breast cancer. Cancer Treat Rev 2010;36:451-7. [Crossref] [PubMed]
- Gucalp A, Traina TA, Eisner JR, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat 2019;173:37-48. [Crossref] [PubMed]
- Liu N, Johnson KJ, Ma CX. Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin Breast Cancer 2018;18:e997-e1002. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Arslan UY, Oksüzoğlu B, Ozdemir N, et al. Outcome of non-metastatic male breast cancer: 118 patients. Med Oncol 2012;29:554-60. [Crossref] [PubMed]
- Johansson I, Nilsson C, Berglund P, et al. Gene expression profiling of primary male breast cancers reveals two unique subgroups and identifies N-acetyltransferase-1 (NAT1) as a novel prognostic biomarker. Breast Cancer Res 2012;14:R31. [Crossref] [PubMed]
- Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet 2006;367:595-604. [Crossref] [PubMed]
- DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin 2014;64:52-62. [Crossref] [PubMed]
- Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol 2014;10:2293-301. [Crossref] [PubMed]
- Haldosén LA, Zhao C, Dahlman-Wright K. Estrogen receptor beta in breast cancer. Mol Cell Endocrinol 2014;382:665-72. [Crossref] [PubMed]
- Kurebayashi J, Moriya T, Ishida T, et al. The prevalence of intrinsic subtypes and prognosis in breast cancer patients of different races. Breast 2007;16:S72-7. [Crossref] [PubMed]
- Pich A, Margaria E, Chiusa L. Oncogenes and male breast carcinoma: c-erbB-2 and p53 coexpression predicts a poor survival. J Clin Oncol 2000;18:2948-56. [Crossref] [PubMed]
- Wang-Rodriguez J, Cross K, Gallagher S, et al. Male breast carcinoma: correlation of ER, PR, Ki-67, Her2-Neu, and p53 with treatment and survival, a study of 65 cases. Mod Pathol 2002;15:853-61. [Crossref] [PubMed]
- Leone JP, Leone J, Zwenger AO, et al. Prognostic significance of tumor subtypes in male breast cancer: a population-based study. Breast Cancer Res Treat 2015;152:601-9. [Crossref] [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [Crossref] [PubMed]
- Visram H, Kanji F, Dent SF. Endocrine therapy for male breast cancer: rates of toxicity and adherence. Curr Oncol 2010;17:17-21. [Crossref] [PubMed]
- Pemmaraju N, Munsell MF, Hortobagyi GN, et al. Retrospective review of male breast cancer patients: analysis of tamoxifen-related side-effects. Ann Oncol 2012;23:1471-4. [Crossref] [PubMed]
- Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer 1994;74:74-7. [Crossref] [PubMed]
- Hayashi H, Kimura M, Yoshimoto N, et al. A case of HER2-positive male breast cancer with lung metastases showing a good response to trastuzumab and paclitaxel treatment. Breast Cancer 2009;16:136-40. [Crossref] [PubMed]
- Chavez-Macgregor M, Clarke CA, Lichtensztajn D, et al. Male breast cancer according to tumor subtype and race: a population-based study. Cancer 2013;119:1611-7. [Crossref] [PubMed]
- Restrepo DJ, Boczar D, Huayllani MT, et al. Survival Disparities in Male Patients With Breast Cancer. Anticancer Res 2019;39:5669-74. [Crossref] [PubMed]
Cite this article as: Wang M, Liu D, Zhang Z, Dai X, Chen G, Zhu L. Molecular subtypes predict the prognosis of male breast cancer: a retrospective cohort study. Transl Breast Cancer Res 2023;4:4.


