
Primary results of ELAINA: a randomized, multicenter, open-label, phase III study of the efficacy and safety of trastuzumab emtansine vs. lapatinib plus capecitabine in Chinese patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy
Highlight box
Key findings
• The ELAINA bridging study met is primary endpoint by showing a 15% reduction in risk of disease progression or death with T-DM1 versus lapatinib plus capecitabine in Chinese women with previously treated HER2-positive mBC. As seen in other studies with T-DM1 in Asian patients, the incidence of grade ≥3 thrombocytopenia was higher with T-DM1 than lapatinib plus capecitabine, but there was no grade ≥3 hemorrhage.
What is known and what is new?
• While the safety and efficacy of T-DM1 has been demonstrated in global populations, prior to this study there had not been a randomized, phase III study of T-DM1 exclusively in Chinese patients.
• These data show that T-DM1 provides antitumor efficacy with an acceptable safety profile in Chinese patients.
What is the implication, and what should change now?
• T-DM1 provides an alternative, chemotherapy-free treatment option for Chinese patients with previously treated HER2-positive mBC.
Introduction
Human epidermal growth factor receptor 2 (HER2)-targeted agents represent the mainstay of therapy for HER2-positive breast cancer. For patients with locally advanced breast cancer (LABC) or metastatic breast cancer (mBC), the standard first-line treatment consists of the monoclonal antibodies pertuzumab and trastuzumab plus chemotherapy (1-3). However, resistance to trastuzumab frequently develops, and this happens through one or multiple mechanisms, including alterations in the target receptor or the downstream signaling pathway (4-7).
Antibody-drug conjugates (ADCs) are a therapeutic modality consisting of a monoclonal antibody that is highly specific for a protein expressed on cancer cells and a cytotoxic moiety joined by a specialized linker. ADCs are designed to target the cytotoxic agent directly to the cancer cell. In this way, highly potent cytotoxic agents can be used without concomitant increases in systemic toxicity, thus improving the therapeutic index. Trastuzumab emtansine (T-DM1) is an ADC that incorporates the HER2-targeted antitumor properties of trastuzumab with the cytotoxic activity of the microtubule inhibitor DM1, thereby allowing intracellular drug delivery specifically to HER2-overexpressing cells (8). T-DM1 is approved in many countries for the treatment of HER2-positive early and advanced breast cancer, and it was recently approved in China for previously treated mBC. Other recent treatment approvals outside China for previously treated HER2-positive mBC include the tyrosine kinase inhibitor (TKI) tucatinib plus trastuzumab and capecitabine (9), the TKI neratinib plus capecitabine (10), the monoclonal HER2-targeted antibody margetuximab plus chemotherapy (11), the HER2-targeted ADC trastuzumab deruxtecan (12), and the subcutaneous trastuzumab formulation plus pertuzumab (13).
For several years, the second-line therapy of choice for Chinese women with HER2-positive mBC was lapatinib plus capecitabine (14). In 2018, the pan-ErbB receptor TKI pyrotinib was approved in China as a second-line treatment for HER2-positive mBC (15), supported by data from two phase III studies (16,17). In one of these trials, PHOEBE, pyrotinib demonstrated improved efficacy vs. lapatinib (each combined with capecitabine), but with increased severe toxicity (16). Head-to-head comparisons of pyrotinib with other approved treatments for previously treated mBC, such as T-DM1, have not been conducted in randomized trials. However, a small real-world study suggests that pyrotinib may result in better clinical outcomes than T-DM1 in some groups of patients. In the global phase III EMILIA trial, T-DM1 significantly prolonged progression-free survival (PFS) and overall survival (OS) with less toxicity than lapatinib plus capecitabine (18,19). As an ADC, T-DM1 is a novel treatment option that could offer an enhanced benefit-risk profile vs. standard TKIs for patients with trastuzumab-resistant mBC in China. However, the global patient population of EMILIA was predominantly (74%) White (18,19), thereby limiting extrapolation of the results to other racial and ethnic groups, including Chinese patients. Moreover, the increased incidence of thrombocytopenia with T-DM1 seen in Asian vs. non-Asian patients (20-22) warranted further investigation of safety in a Chinese population.
The phase III ELAINA bridging trial was undertaken to compare T-DM1 with lapatinib plus capecitabine in Chinese patients with trastuzumab- and taxane-pretreated LABC/mBC. The aim was to determine whether the benefit of T-DM1 in Chinese patients is consistent with that observed in the global population of EMILIA. This article is presented in accordance with the CONSORT reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-2/rc).
Methods
Study design
ELAINA (NCT03084939) was a two-stage, two-arm, randomized, multicenter, open-label bridging study. In stage 1, patients were randomized (3:1) to receive T-DM1 or lapatinib plus capecitabine. An interactive voice/Web response system (IxRS) was used to randomize patients. A permuted block randomization scheme was used to stratify patients according to the following factors: number of prior chemotherapeutic regimens for unresectable LABC or mBC (0–1 vs. >1) and visceral vs. nonvisceral disease. A bridging design was implemented to allow comparison of the treatment benefit in patients from China with the benefit observed in the EMILIA trial, which included a predominantly White patient population. Stage 2 comprised a single-arm safety cohort in which patients received T-DM1 only, to enable the safety profile of T-DM1 to be sufficiently characterized in Chinese patients. The stage 1 findings are presented here.
Approximately 350 eligible patients were planned to be enrolled from 25 sites, to result in 150 and 50 patients in the stage 1 T-DM1 and control arms, respectively, and 150 patients in the stage 2 single-arm T-DM1 safety extension (achieving a total of 300 patients treated with T-DM1).
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice (GCP) guidelines. The study protocol was approved by the institutional review board or the independent ethics committee at each study site. All participants provided written informed consent.
Eligibility criteria
Eligibility criteria included histologically or cytologically confirmed, measurable [by modified Response Evaluation Criteria in Solid Tumors (RECIST)] or nonmeasurable unresectable LABC/mBC; progression during or after the most recent treatment for LABC/mBC or within 6 months after treatment for early-stage disease; centrally confirmed HER2-positive status [immunohistochemistry 3+ and/or gene amplified (HER2 to CEP 17 ratio ≥2) by in situ hybridization]; prior treatment with both a taxane and trastuzumab for breast cancer; left ventricular ejection fraction ≥50% by echocardiogram or multiple-gated acquisition; Eastern Cooperative Oncology Group performance status ≤1; and adequate hematologic and organ function.
Major exclusion criteria included prior treatment with lapatinib, capecitabine, or T-DM1; grade ≥3 peripheral neuropathy [per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (23)]; history of treatment with anticancer therapy (not including hormonal therapy) or investigational agents within 21 days, or radiation therapy within 14 days, prior to randomization (stage 1) or enrollment (stage 2); and brain metastases that were untreated or required therapy to control symptoms within 30 days before randomization (stage 1) or enrollment (stage 2).
Study assessments
Baseline clinical characteristics were assessed by the investigator at screening. Tumor assessments were conducted every 6 weeks from cycle 1 day 1 for the first 78 weeks and every 12 weeks thereafter, until investigator-assessed disease progression or study termination. Patients could continue study treatment until disease progression, unmanageable toxicity, or study termination. After disease progression, the frequency, method, and evaluation criteria of tumor assessments were per investigators’ routine clinical practice. Patients who demonstrated control of their systemic disease while receiving T-DM1 but who developed isolated brain metastases that were treatable with radiation, were allowed to continue with T-DM1 until systemic disease progression. Approximately 30 days after study termination, patients returned for a follow-up visit. They continued to be followed every 3 months for OS and documentation of subsequent anti-cancer treatments. Follow-up continued until death, loss to follow-up, withdrawal of consent, or study discontinuation by the sponsor.
Study objectives
The primary objective was to demonstrate consistency of the safety and efficacy data with those from the EMILIA global study, by evaluating the PFS of T-DM1 compared with lapatinib plus capecitabine. PFS was defined as the time from randomization to the first occurrence of investigator-determined disease progression by RECIST v1.1, or death from any cause, whichever occurred first.
Secondary efficacy endpoints included investigator-assessed objective response rate (ORR) per RECIST v1.1, duration of response, and OS. Health-related quality of life (HRQoL) and pharmacokinetic (PK) parameters and were also evaluated. HRQoL was measured with the Functional Assessment of Cancer Therapy-Breast (FACT-B). The FACT-B is a patient-reported questionnaire that has been validated in patients with breast cancer [Brady 1997 (24)]. It contains 5 subscales: physical, social, emotional, functional well-being, and breast-cancer specific items. It was administered to patients at baseline (cycle 1, day 1), on day 1 of every other treatment cycle, and at the treatment discontinuation visit. Samples for PK analysis were collected before drug administration and 15 to 30 minutes after infusion during cycles 1–4. The primary PK analytes were T-DM1, total trastuzumab, and DM1.
Study treatment
T-DM1 (3.6 mg/kg) was administered intravenously on day 1 of a 3-week cycle. The first T-DM1 infusion was given over 90 (±10) minutes, with subsequent doses infused over 30 (±10) minutes. Infusion interruptions and rate adjustments were permitted for patients experiencing infusion-associated symptoms. Lapatinib (1,250 mg) was taken orally, ≥1 h before or ≥1 h after a meal, once daily on days 1−21. The total daily dose of capecitabine was 2,000 mg/m2 (two oral doses of 1,000 mg/m2 administered ~12 h apart, with food or ≤30 minutes after food) on days 1−14 in a repeating 21-day cycle. The cycle was defined by the start of capecitabine chemotherapy.
Statistics
The intent-to-treat (ITT) population included all randomized patients from stage 1. Efficacy was analyzed in the ITT population. The safety population comprised all patients from stage 1 who received at least one dose of study drug. Patients were included in the PK analysis if they had received at least one dose of T-DM1 and had at least one post-dose serum or plasma result by the data cut-off time for the primary analysis.
The trial was designed with the prespecified number of events to have 80% probability to detect a hazard ratio (HR) of ≤0.825, which maintained 50% of the risk reduction determined in EMILIA. The sample size estimation was based on the assumption of a median PFS of 6.4 months in the control arm and an HR of 0.65, as well as a recruitment period of approximately 13 months. A two-sided stratified log-rank test was performed on the PFS data. However, since the objective was to determine consistency between its data and those in EMILIA, this bridging study was not fully powered to demonstrate statistically significant differences between the treatment arms, thus all analyses are descriptive.
Baseline comparability between treatment groups was evaluated based on medians and frequencies of demographic and disease characteristics, medical history, and treatment history. For the primary analysis of PFS, data for patients without disease progression or death from any cause at the time of the data cutoff were censored at the time of the last valid tumor assessment or, if no tumor assessment was performed after the baseline visit, at the time of randomization plus 1 day. Data from patients who were lost to follow-up were included in the analysis as censored observations on the last tumor assessment date that the patient was known to be progression-free.
Only patients with measurable disease at baseline were included in the ORR analysis. Patients without a postbaseline tumor assessment were categorized as nonresponders. An ORR estimate and 95% confidence interval (CI) were calculated for each treatment arm.
The Kaplan-Meier method was used to estimate the median PFS, duration of response, OS, and time to deterioration in HRQoL (and the corresponding 95% CIs) for each treatment group. Deterioration in HRQoL was defined as a clinically meaningful (i.e., ≥5-point change) (25) decrease in the FACT-B Trial Outcome Index, which comprises physical well-being, functional well-being, and breast cancer symptom subscales (24). For PFS and OS, a Cox proportional hazards model, stratified by number of prior chemotherapeutic regimens for unresectable LABC or mBC (0–1 vs. >1) and visceral vs. nonvisceral disease, was used to estimate the HRs and 95% CIs between the treatment arms. Forest plots (including estimated HRs and 95% CIs) examined PFS across clinically relevant subgroups.
Results
Between April 2017 and October 2018, 200 patients with HER2-positive LABC/mBC were enrolled at 19 centers in China and randomized to T-DM1 (n=151) or lapatinib plus capecitabine (n=49) (Figure 1). Baseline patient demographics and disease characteristics were generally balanced between the two groups (Table 1). The only exceptions to this were that more patients in the lapatinib plus capecitabine arm vs. the T-DM1 arm had de novo mBC (30.6 vs. 13.9%), and more patients in the T-DM1 arm vs. the lapatinib plus capecitabine arm had undergone curative breast cancer surgery (85.4% vs. 69.4%) and/or radiotherapy (61.6% vs. 53.1%).
Table 1
| Characteristic | Lapatinib + capecitabine (n=49) | T-DM1 (n=151) |
|---|---|---|
| Median age, years [range] | 52 [28–66] | 51 [28–78] |
| ECOG performance status, n (%) | ||
| 0 | 26 (53.1) | 83 (55.0) |
| 1 | 23 (46.9) | 68 (45.0) |
| Visceral disease, n (%) | ||
| Yes | 37 (75.5) | 117 (77.5) |
| No | 12 (24.5) | 34 (22.5) |
| Measurable disease, n (%) | 43 (87.8) | 117 (77.5) |
| Hormone receptor-positive at initial diagnosis, n (%) | 25 (51.0) | 68 (45.0) |
| Prior systemic therapy, n (%) | ||
| (Neo)adjuvant only | 12 (24.5) | 35 (23.2) |
| Metastatic only | 15 (30.6) | 21 (13.9) |
| (Neo)adjuvant + metastatic | 22 (44.9) | 95 (62.9) |
| Prior (neo)adjuvant treatment, n (%) | ||
| Hormonal | 14 (28.6) | 43 (28.5) |
| Anthracycline | 29 (59.2.2) | 121 (80.1) |
| Taxane | 31 (63.3) | 116 (76.8) |
| Other chemotherapy | 28 (57.1) | 120 (79.5) |
| Pertuzumab | 2 (4.1) | 1 (0.7) |
| Trastuzumab | 17 (34.7) | 71 (47.0) |
| Prior treatment for metastatic disease, n (%) | ||
| Hormonal | 9 (18.4) | 33 (21.9) |
| Anthracycline | 4 (8.2) | 21 (13.9) |
| Taxane | 32 (65.3) | 77 (51.0) |
| Other chemotherapy | 21 (42.9) | 58 (38.4) |
| Pertuzumab | 7 (14.3) | 18 (11.9) |
| Trastuzumab | 37 (75.5) | 109 (72.2 |
| Prior trastuzumab setting, n (%) | ||
| (Neo)adjuvant only | 12 (24.5) | 42 (27.8) |
| Metastatic only | 32 (65.3) | 80 (53.0) |
| (Neo)adjuvant + metastatic | 5 (10.2) | 29 (19.2) |
| Median time since last trastuzumab, months (range) | 1.61 (0.7–26.6) | 2.23 (0.4–48.6) |
| Median duration of prior trastuzumab, months (range) | 7.92 (0.7–18.7) | 8.74 (0.7–33.6) |
| Prior curative breast cancer surgery, n (%) | 34 (69.4) | 129 (85.4) |
| Prior radiotherapy, n (%) | 26 (53.1) | 93 (61.6) |
T-DM1, trastuzumab emtansine; ECOG, Eastern Cooperative Oncology Group; (neo)adjuvant, neoadjuvant or adjuvant.
Efficacy
The data cut-off date for all analyses, except for OS, was October 8, 2018 [median duration of follow-up was 9.7 (range, 3.4–14.4) months in the lapatinib plus capecitabine arm and 8.6 (range, 1.0–16.8) months in the T-DM1 arm]. The data cut-off date for the OS analysis (i.e., when there were ≥50% of expected OS events) was July 12, 2021 [median duration of follow-up was 34.0 (range, 3.4–46.7) months in the lapatinib plus capecitabine arm and 27.6 (range, 1.0–47.8) months in the T-DM1 arm].
T-DM1 treatment was associated with a 15% reduction in the risk of disease progression or death compared with lapatinib plus capecitabine in trastuzumab- and taxane-pretreated Chinese patients (stratified HR =0.85; 95% CI: 0.56–1.29). Median duration of PFS was similar with T-DM1 and lapatinib plus capecitabine (7.0 vs. 6.8 months, respectively). The Kaplan-Meier curves separated at approximately 7 months and remained separated from then onward (Figure 2A). This benefit was consistently observed in most clinically relevant subgroups (Figure 2B).
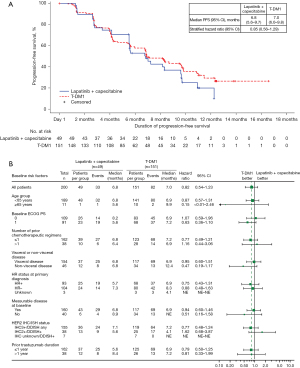
Approximately 80% of patients in both treatment arms (117 and 43 patients in the T-DM1 and lapatinib plus capecitabine arms, respectively) had measurable disease at baseline and were therefore included in the ORR analysis. Similar proportions of patients achieved an objective response in the T-DM1 and the lapatinib plus capecitabine arms (50.4% and 55.8%, respectively; Table 2). Duration of response was the same (8.4 months) in both treatment arms (Table 2).
Table 2
| Endpoint | Lapatinib + capecitabine (n=43) | T-DM1 (n=117) |
|---|---|---|
| ORR, n (%) | 24 (55.8) | 59 (50.4) |
| 95% CI | 39.9–70.9 | 41.0–59.8 |
| Complete response, n (%) | 1 (2.3) | 3 (2.6) |
| 95% CI | 0.06–12.3 | 0.5–7.3 |
| Partial response, n (%) | 23 (53.5) | 56 (47.9) |
| 95% CI | 37.7–68.8 | 38.5–57.3 |
| Stable disease, n (%) | 10 (23.3) | 35 (29.9) |
| 95% CI | 11.8–38.6 | 21.8–39.1 |
| Median DOR, months | 8.4 | 8.4 |
| 95% CI | 5.5–8.4 | 5.5–NE |
| Median OS, months | 40.0 | 33.2 |
| 95% CI | 23.6–NE | 27.6–43.9 |
| Hazard ratio (95% CI) | 1.08 (0.69–1.69) | |
T-DM1, trastuzumab emtansine; ORR, objective response rate; CI, confidence interval; DOR, duration of response; OS, overall survival; NE, not evaluable.
At the data cut-off date of July 12, 2021, 103 deaths were reported [77 (51.0%) and 26 (53.1%) in the T-DM1 and lapatinib plus capecitabine arms, respectively]. OS was similar between the two groups (HR =1.08; 95% CI: 0.69–1.69).
Health-related quality of life
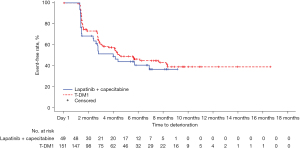
HRQoL was analyzed in the patients from the ITT population who had a baseline and at least one post-baseline assessment (T-DM1 n=151; lapatinib + capecitabine n=49). A small numerical difference was observed in the median time to deterioration in HRQoL (as measured by a ≥5-point decrease from baseline score in the FACT-B Trial Outcome Index) between treatment arms (HR =0.76; 95% CI: 0.49–1.20). The Kaplan-Meier estimated median time to deterioration was 4.4 and 4.0 months in the T-DM1 and lapatinib plus capecitabine arms, respectively, with a separation between the Kaplan-Meier curves at approximately 2 months in favor of T-DM1, which was maintained from then onwards (Figure 3).
Safety
Treatment exposure was similar between the treatment arms (Table 3). Any-grade adverse events (AEs) occurred in 98.0% and 100% of patients in the T-DM1 and lapatinib plus capecitabine arms, respectively. A similar incidence of grade ≥3 AEs (54.3% and 57.1%) and serious AEs (SAEs; 20.5% and 20.4%) occurred with T-DM1 and lapatinib plus capecitabine, respectively (Table 4). More patients in the T-DM1 arm had AEs requiring study treatment withdrawal (11.9% vs. 4.1% with lapatinib plus capecitabine). Fewer patients in the T-DM1 arm had AE-related dose reductions (11.3% vs. 49.0% with lapatinib plus capecitabine).
Table 3
| Exposure | Lapatinib + capecitabine (n=49) | T-DM1 (n=151) | ||
|---|---|---|---|---|
| Lapatinib | Capecitabine | All-grade | ||
| Cycles received, n (%) | ||||
| ≤6 | 15 (30.6) | 15 (30.6) | 63 (41.7) | |
| 7–12 | 21 (42.9) | 21 (42.9) | 53 (35.1) | |
| 13–18 | 12 (24.5) | 12 (24.5) | 29 (19.2) | |
| 19–24 | 1 (2.0) | 1 (2.0) | 5 (3.3) | |
| >24 | 0 | 0 | 1 (0.7) | |
| Median treatment duration, months [range] | 6.1 [1–14] | 6.4 [1–14] | 5.5 [0–17] | |
T-DM1, trastuzumab emtansine.
Table 4
| Adverse event | Lapatinib + capecitabine (n=49), n (%) | T-DM1 (n=151), n (%) |
|---|---|---|
| Any-grade AE | 49 (100.0) | 148 (98.0) |
| Grade ≥3 AE | 28 (57.1) | 82 (54.3) |
| Serious AE | 10 (20.4) | 31 (20.5) |
| AE necessitating treatment withdrawal | 2 (4.1) | 18 (11.9) |
| AE necessitating treatment interruption | 3 (26.5) | 35 (23.2) |
| AE necessitating treatment dose reduction | 24 (49.0) | 17 (11.3) |
T-DM1 trastuzumab emtansine; AE, adverse event.
Consistent with the known safety profile of T-DM1 (26), higher incidences of thrombocytopenia, hemorrhage, infusion-related reactions (IRRs), cardiac dysfunction, and pneumonitis occurred in the T-DM1 arm vs. the lapatinib plus capecitabine arm (Table 5). All-grade thrombocytopenia occurred in 76.2% and 20.4% of patients in the T-DM1 and lapatinib plus capecitabine arms, respectively, and grade ≥3 thrombocytopenia occurred in 40.4% and 4.1% of patients, respectively. The combined preferred terms of thrombocytopenia and decreased platelet count represented the most common SAE and AE leading to dose discontinuation in the T-DM1 arm. In three patients, thrombocytopenia did not recover to grade ≤2 after T-DM1 was discontinued for >90 days. These patients were treated with a thrombopoietin receptor agonist after hematology consultation, and platelet counts recovered in one patient.
Table 5
| Selected AEs† | Lapatinib + capecitabine (n=49), n (%) | T-DM1 (n=151), n (%) | |||
|---|---|---|---|---|---|
| All-grade | Grade ≥3 | All-grade | Grade ≥3 | ||
| Any selected AE | 44 (89.8) | 4 (8.2) | 139 (92.1) | 65 (43.0) | |
| Hepatoxicity | 41 (83.7) | 1 (2.0) | 119 (78.8) | 13 (8.6) | |
| Thrombocytopenia | 10 (20.4) | 2 (4.1) | 115 (76.2) | 61 (40.4) | |
| Infusion-related reaction/hypersensitivity | 0 | 0 | 12 (7.9) | 1 (0.7) | |
| Pneumonitis | 0 | 0 | 1 (0.7) | 0 | |
| Cardiac dysfunction | 0 | 0 | 3 (2.0) | 1 (0.7) | |
| Peripheral neuropathy | 3 (6.1) | 1 (2.0) | 12 (7.9) | 0 | |
| Hemorrhage | 2 (4.1) | 0 | 49 (32.5) | 0 | |
†, AEs selected based on the known safety profile of T-DM1 and defined by related preferred terms for each AE category. AE, adverse event; T-DM1 trastuzumab emtansine.
Hemorrhage was more frequent with T-DM1 (32.5%) than with lapatinib plus capecitabine (4.1%), but no grade ≥3 events occurred in either arm. The most common hemorrhage AEs in the T-DM1 arm were epistaxis and gingival bleeding. Infusion reaction and hypersensitivity AEs occurred with T-DM1 only (7.9%), most commonly IRRs (6.9%) and pyrexia (2.0%). A single (0.7%) grade 3 IRR event occurred. Cardiac function AEs occurred in the T-DM1 arm only (2.0%) and included grade 3 cardiac failure (0.7%), grade 1 left ventricular dysfunction (0.7%), and grade 2 right ventricular dysfunction (0.7%). Left ventricular ejection fraction assessment results were consistent between treatment arms. One (0.7%) grade 1 pneumonitis AE (interstitial lung disease) occurred in the T-DM1 arm. There were no pneumonitis AEs of grade ≥2 severity with either treatment.
PKs
Serum T-DM1 concentrations are summarized in Table S1. The mean concentration ± standard deviation (SD) at 15 to 30 minutes post-infusion was 76.4±26.9 and 69.7±27.4 µg/mL for cycles 1 and 4, respectively. Minimum T-DM1 accumulation was observed over cycles 1 through 4 upon repeated T-DM1 treatment and the steady state concentration was reached at cycle 1. Serum total trastuzumab concentrations are summarized in Table S2. The mean concentration ± SD at 15 to 30 minutes post-infusion was 80.4±23.9 and 77.3±27.9 µg/mL for cycles 1 and 4, respectively. Minimum exposure accumulation was observed over cycles 1 through 4 upon repeated T-DM1 treatment. Plasma DM1 concentrations are summarized in Table S3. The mean concentration ± SD at 15 to 30 minutes post-infusion was 14.0±7.1 and 13.9±6.3 µg/mL for cycles 1 and 4, respectively, suggesting that DM1 did not accumulate over cycles 1 through 4 upon repeated T-DM1 treatment.
Discussion
Until recently, the Chinese Society of Clinical Oncology (CSCO) recommended lapatinib plus capecitabine for the management of patients with trastuzumab-resistant HER2-positive mBC. In 2020, updated CSCO guidelines recommended pyrotinib plus capecitabine as the standard of care for these patients (27). This decision was based on results of the PHOEBE and PHENIX trials, which demonstrated significantly increased PFS with pyrotinib plus capecitabine vs. lapatinib plus capecitabine and placebo plus capecitabine regimens (16,17). T-DM1 has demonstrated significant OS and PFS benefits vs. lapatinib plus capecitabine in taxane- and trastuzumab-pretreated patients with LABC/mBC (18,19); despite this, TKI-based regimens with pyrotinib or lapatinib remained the gold standard in this population in China (14,27).
The current study, ELAINA, was designed to bridge the efficacy and safety data from the global EMILIA study evaluating T-DM1 vs. lapatinib plus capecitabine (the standard of care at study initiation) to a Chinese population. The bridging design used in ELAINA is well established for evaluating whether treatment effects in Chinese patients are comparable to those in global pivotal studies, and is widely used in China for registration purposes. The reduced time to data availability (vs. fully powered studies) may allow earlier approval and patient access to a therapy that has demonstrated significant and meaningful benefit in a global population. However, this design is limited in that the reduced patient numbers result in reduced statistical power to thoroughly define statistical differences between treatment arms. In addition, this study was only performed in mainland China, and was not a multi-regional clinical study (MRCT).
The primary objective of ELAINA was met, i.e., it demonstrated consistency with the EMILIA results. T-DM1 prolonged PFS compared with lapatinib plus capecitabine in trastuzumab- and taxane-pretreated Chinese patients with HER2-positive LABC/mBC. Benefit was observed, regardless of the line of therapy, in patients with a disease-free interval of <6 months after completing trastuzumab-based therapy in the adjuvant or neoadjuvant setting; benefit was unaffected by the presence of visceral disease. The magnitude of PFS benefit with T-DM1 vs. lapatinib plus capecitabine (stratified HR =0.85; 95% CI: 0.56–1.29) was generally consistent with that observed in the EMILIA ITT (HR =0.65; 95% CI: 0.549–0.771) (18,19) and Asian (HR =0.72; 95% CI: 0.48–1.02) (21) populations. The risk of death was similar between the treatment arms (HR =1.08; 95% CI: 0.69–1.69). Importantly, while median OS was comparable for the T-DM1 arms in ELAINA (33.2 months) and EMILIA (30.9 months) (18), median OS with lapatinib plus capecitabine in ELAINA (40.0 months) was substantially higher than that expected based on the lapatinib results in EMILIA (25.1 months). Median time to deterioration in HRQoL from baseline was slightly longer in the T-DM1 arm.
PK data of the primary analytes in ELAINA were consistent with previous findings in T-DM1-treated patients with mBC (28). These analyses have shown that T-DM1 has similar PK across ethnicities (White, Asian, other) and that the recommended dosing regimen is suitable for different ethnic groups (29).
T-DM1 was generally well tolerated with an acceptable safety profile. Incidences of grade ≥3 AEs and SAEs were similar between the treatment arms. More patients receiving T-DM1 vs. those on lapatinib plus capecitabine had AEs necessitating study treatment withdrawal (11.9% vs. 4.1%), while more patients in the lapatinib plus capecitabine arm had AEs requiring dose reductions (49.0% vs. 11.3%). Consistent with the known safety profile of T-DM1 (26), hemorrhage, IRRs, cardiac dysfunction, and pneumonitis AEs were numerically increased with T-DM1 compared with lapatinib plus capecitabine. However, most events were grade 1 or 2 in severity, except for one case of grade 3 IRR and one of grade 3 cardiac failure.
All-grade and grade ≥3 thrombocytopenia increased with T-DM1 compared with lapatinib plus capecitabine. The incidence of thrombocytopenia AEs in Chinese patients in ELAINA (the current study), is higher than that reported in studies with global populations, including EMILIA (18,19). This is consistent with prior studies demonstrating an increased risk of thrombocytopenia in Asian patients vs. non-Asian patients (20-22,30). In a pooled analysis of six studies of T-DM1 in patients with HER2-positive mBC, Asian patients demonstrated a higher incidence of grade ≥3 decreased platelet count than non-Asian patients (44.4% vs. 10.6%) (20). A similar difference was observed in Asian patients (43.8%) vs. the global population of EMILIA (13.9%) (21). The results from ELAINA are also consistent with the international, single-arm, phase IIIb KAMILLA trial of T-DM1 in HER2-positive mBC, in which grade ≥3 thrombocytopenia occurred in 44.7% and 3.7% of Asian and non-Asian patients, respectively (22). In these studies (20-22), thrombocytopenia in Asian patients was not associated with an increased incidence of clinically significant hemorrhage and could generally be managed with the T-DM1 dose adjustments recommended for the global population (29).
In China, recombinant human thrombopoietin (rhTPO) is a standard treatment for chemotherapy-induced thrombocytopenia, but it is not available elsewhere (31,32). rhTPO use is associated with the generation of antibodies against endogenous thrombopoietin, which results in refractory thrombocytopenia (33). Taxanes also induce thrombocytopenia, and prior taxane treatment was required for study entry in ELAINA. Chinese patients may have received prior rhTPO to manage taxane-induced thrombocytopenia (34). Caution should be exercised when using rhTPO to manage thrombocytopenia in patients treated with T-DM1, and these patients should be carefully monitored.
The T-DM1 benefit-risk profile in ELAINA is consistent with previous clinical trials (18,19,35,36) and real-world data in patients with pretreated LABC/mBC, including Chinese patients (37-41). A recent network meta-analysis suggested that pyrotinib plus capecitabine may be the most efficacious treatment among the nine regimens (including T-DM1) evaluated for patients with previously treated HER2-positive mBC (42). It also found that pyrotinib plus capecitabine may be associated with more grade ≥3 AEs while T-DM1 most likely had the lowest incidence of grade ≥3 AEs. This is in keeping with the expectation that T-DM1 exercises most of its activity by selectively releasing a cytotoxic payload, DM1, to cancer cells while limiting exposure to normal tissues. The meta-analysis had several limitations, including the lack of randomized clinical studies comparing pyrotinib plus capecitabine vs. T-DM1 (42). The single intravenous administration of T-DM1 once every 3 weeks, vs. a daily (pyrotinib) and twice-daily (capecitabine) administration schedule, may be preferred by some patients (16-18). Furthermore, the rate of grade ≥3 diarrhea with pyrotinib (31%) is substantially higher than with most oral TKIs, and this may limit treatment adherence (43,44).
Recent data from the phase III DESTINY-Breast03 study further support the use of HER2-directed ADCs in advanced breast cancer (45). Trastuzumab deruxtecan resulted in a substantially and statistically significantly improved PFS compared with T-DM1 as second-line treatment for HER2-positive mBC [median not reached (95% CI: 18.5 to not evaluable) vs. 6.8 (95% CI: 5.6–8.2) months; HR =0.28; 95% CI: 0.22–0.37]. OS was also significantly improved with trastuzumab deruxtecan (HR =0.55; 95% CI: 0.36–0.86). In addition, trastuzumab deruxtecan was associated with a higher incidence of AE-related dose reduction (21.4% vs. 12.6%), AE-related study treatment discontinuation (13.6% vs. 7.3%), and grade ≥3 neutropenia (19.1% vs. 3.1%), while T-DM1 was associated with more grade ≥3 thrombocytopenia (24.9% vs. 7.0% with trastuzumab deruxtecan). Trastuzumab deruxtecan may offer an additional non-TKI treatment option in the second-line setting, but it is not currently approved in China.
Since resistance eventually develops with all therapies increasing the number of treatment options for HER2-positive, previously treated mBC may result in prolonged OS and improved HRQoL. Resistance mechanisms for T-DM1 include, but are not limited to, loss of HER2 expression and intracellular alterations that block the release of DM1 and/or reduce DM1 cytotoxicity. Understanding the optimal sequence of therapies is an important next step in continuing to improve efficacy. Treatment options in development across the HER2-positive breast cancer spectrum include single-agent ADCs with novel linker and payload technology, and combining ADCs with other agents such as checkpoint inhibitors (e.g., atezolizumab) or TKIs (e.g., tucatinib) (1,46-51). Evaluation of the long-term efficacy and safety of T-DM1 and other therapies in this setting is also of interest.
Conclusions
The ELAINA study met its primary objective. The data confirm that the benefit-risk profile of T-DM1 is acceptable in Chinese patients with HER2-positive LABC/mBC who have received prior trastuzumab and taxane therapy. T-DM1 provides an efficacious and tolerable chemotherapy-free second-line treatment option for mBC.
Acknowledgments
We gratefully acknowledge the patients who participated in this study and the clinical site teams. Medical writing assistance was provided by Tracy McNally, PhD, and Holly Strausbaugh, PhD, on behalf of Twist Medical, and was funded by F. Hoffmann-La Roche.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist, available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-2/rc
Data Sharing Statement: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-2/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-2/coif). FW, FL, and ER are employees of and hold stock in F. Hoffmann-La Roche. XW serves as an unpaid editorial board member of Translational Breast Cancer Research from December 2022 to November 2024. QZ, YY serves as unpaid editorial board members of Translational Breast Cancer Research from March 2022 to February 2024. ZJ serves as the Editor-in-Chief of Translational Breast Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice (GCP) guidelines. The study protocol was approved by the institutional review board or the independent ethics committee at each study site. All participants provided written informed consent to participate in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martínez-Sáez O, Prat A. Current and Future Management of HER2-Positive Metastatic Breast Cancer. JCO Oncol Pract 2021;17:594-604. [Crossref] [PubMed]
- Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021;32:1475-95. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology – Breast cancer, version 2.2022. December 20, 2021.
- Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012;487:505-9. [Crossref] [PubMed]
- Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004;6:117-27. [Crossref] [PubMed]
- Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 2007;12:395-402. [Crossref] [PubMed]
- Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 2001;93:1852-7. [Crossref] [PubMed]
- Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011;128:347-56. [Crossref] [PubMed]
- SeaGen, Inc. TUKYSA® (tucatinib) tablets, for oral use. Prescribing information, 2021.
- Puma Biotechnology, Inc. NERLYNX® (neratinib) tablets, for oral use. Prescribing information, 2021.
- MacroGenics, Inc. MARGENZATM (margetuximab-cmkb) injection, for intravenous use. Prescribing information, 2020.
- Daiichi Sankyo, Inc. ENHERTU® (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use. Prescribing information, 2021.
- Genentech, Inc. PHESGO® (pertuzumab, trastuzumab, and hyaluronidase-zzxf) injection, for subcutaneous use. Prescribing information, 2020.
- Li J, Jiang Z. CSCO BC guideline: updates for HER2 positive breast cancer in 2020. Transl Breast Cancer Res 2020;1:4. [Crossref]
- Blair HA. Pyrotinib: First Global Approval. Drugs 2018;78:1751-5. [Crossref] [PubMed]
- Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. [Crossref] [PubMed]
- Yan M, Bian L, Hu X, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res 2020;1:13. [Crossref]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [Crossref] [PubMed]
- Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732-42. [Crossref] [PubMed]
- Diéras V, Harbeck N, Budd GT, et al. Trastuzumab emtansine in human epidermal growth factor receptor 2-positive metastatic breast cancer: an integrated safety analysis. J Clin Oncol 2014;32:2750-7. [Crossref] [PubMed]
- Im SA, Park IH, Sohn J, et al. Trastuzumab emtansine in Asian patients with previously treated HER2-positive locally advanced or metastatic breast cancer: data from the phase 3 EMILIA study. Annals Oncol 2021;32:S457-S515. [Crossref]
- Wuerstlein R, Ellis P, Montemurro F, et al. Safety of trastuzumab emtansine (T-DM1) in patients (pts) with HER2-positive locally advanced or metastatic breast cancer (mBC): Final results from KAMILLA Cohorts 1 (global) and 2 (Asia). J Clin Oncol 2021;39:1039. [Crossref]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0;2009.
- Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997;15:974-86. [Crossref] [PubMed]
- Eton DT, Cella D, Yost KJ, et al. A combination of distribution and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol 2004;57:898-910. [Crossref] [PubMed]
- Montemurro F, Ellis P, Anton A, et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: Primary results from the KAMILLA study cohort 1. Eur J Cancer 2019;109:92-102. [Crossref] [PubMed]
- Jiang Z, Song E, Wang X, et al. Guidelines of Chinese Society of Clinical Oncology (CSCO) on diagnosis and treatment of breast cancer (2020 version). Transl Breast Cancer Res 2020;1:27. [Crossref]
- Lu D, Girish S, Gao Y, et al. Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: clinical implications of the effect of covariates. Cancer Chemother Pharmacol 2014;74:399-410. [Crossref] [PubMed]
- Li C, Wang B, Lu D, et al. Ethnic sensitivity assessment of the antibody-drug conjugate trastuzumab emtansine (T-DM1) in patients with HER2-positive locally advanced or metastatic breast cancer. Cancer Chemother Pharmacol 2016;78:547-58. [Crossref] [PubMed]
- Modi ND, Sorich MJ, Rowland A, et al. Predicting Thrombocytopenia in Patients With Breast Cancer Treated With Ado-trastuzumab Emtansine. Clin Breast Cancer 2020;20:e220-8. [Crossref] [PubMed]
- Leader A, Hofstetter L, Spectre G. Challenges and Advances in Managing Thrombocytopenic Cancer Patients. J Clin Med 2021;10:1169. [Crossref] [PubMed]
- Chinese Society of Clinical Oncology Consensus Expert Committee on Thrombocytopenia Induced by Tumor Chemotherapy. Consensus on clinical diagnosis, treatment and prevention management of chemotherapy induced thrombocytopenia in China. Zhonghua Zhong Liu Za Zhi 2018;40:714-20. [PubMed]
- Levy B, Arnason JE, Bussel JB. The use of second-generation thrombopoietic agents for chemotherapy-induced thrombocytopenia. Curr Opin Oncol 2008;20:690-6. [Crossref] [PubMed]
- Hashiguchi Y, Fukuda T, Ichimura T, et al. Chemotherapy-induced thrombocytopenia and clinical bleeding in patients with gynecologic malignancy. Eur J Gynaecol Oncol 2015;36:168-73. [PubMed]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [Crossref] [PubMed]
- Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 2017;18:743-54. [Crossref] [PubMed]
- Conte B, Fabi A, Poggio F, et al. T-DM1 Efficacy in Patients With HER2-positive Metastatic Breast Cancer Progressing After a Taxane Plus Pertuzumab and Trastuzumab: An Italian Multicenter Observational Study. Clin Breast Cancer 2020;20:e181-7. [Crossref] [PubMed]
- Hardy-Werbin M, Quiroga V, Cirauqui B, et al. Real-world data on T-DM1 efficacy - results of a single-center retrospective study of HER2-positive breast cancer patients. Sci Rep 2019;9:12760. [Crossref] [PubMed]
- Vici P, Pizzuti L, Michelotti A, et al. A retrospective multicentric observational study of trastuzumab emtansine in HER2 positive metastatic breast cancer: a real-world experience. Oncotarget 2017;8:56921-31. [Crossref] [PubMed]
- Yardley DA, Krop IE, LoRusso PM, et al. Trastuzumab Emtansine (T-DM1) in Patients With HER2-Positive Metastatic Breast Cancer Previously Treated With Chemotherapy and 2 or More HER2-Targeted Agents: Results From the T-PAS Expanded Access Study. Cancer J 2015;21:357-64. [Crossref] [PubMed]
- Yeo W, Luk MY, Soong IS, et al. Efficacy and tolerability of trastuzumab emtansine in advanced human epidermal growth factor receptor 2-positive breast cancer. Hong Kong Med J 2018;24:56-62. [Crossref] [PubMed]
- Liao H, Huang W, Liu Y, et al. Efficacy and Safety of Pyrotinib Versus T-DM1 in HER2+ Metastatic Breast Cancer Patients Pre-Treated With Trastuzumab and a Taxane: A Bayesian Network Meta-Analysis. Front Oncol 2021;11:608781. [Crossref] [PubMed]
- Rugo HS, Di Palma JA, Tripathy D, et al. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat 2019;175:5-15. [Crossref] [PubMed]
- Secombe KR, Van Sebille YZA, Mayo BJ, et al. Diarrhea Induced by Small Molecule Tyrosine Kinase Inhibitors Compared With Chemotherapy: Potential Role of the Microbiome. Integr Cancer Ther 2020;19:1534735420928493. [Crossref] [PubMed]
- Cortés J, Kim SB, Chung WP, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N Engl J Med 2022;386:1143-54. [Crossref] [PubMed]
- Hurvitz SA, Harbeck N, Vahdat L, et al. 126TiP HER2CLIMB-02: Tucatinib or placebo with T-DM1 for unresectable locally-advanced or metastatic HER2+ breast cancer. Ann Oncol 2021;32:S75. [Crossref]
- Saura Manich C, O'Shaughnessy J, Aftimos PG, et al. Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol 2021;32:S1283-346. [LBA15]. [Crossref]
- Sharma M, Carvajal RD, Hanna GJ, et al. Preliminary results from a phase 1/2study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. J Clin Oncol 2021;39:2549. [Crossref]
- Meric-Bernstam F, Calvo E, Moreno V, et al. A phase I dose escalation study evaluating the safety and tolerability of a novel anti-HER2 antibody-drug conjugate (PF-06804103) in patients with HER2-positive solid tumors. J Clin Oncol 2020;38:1039. [Crossref]
- Hamilton EP, Kaklamani V, Falkson C, et al. Impact of Anti-HER2 Treatments Combined With Atezolizumab on the Tumor Immune Microenvironment in Early or Metastatic Breast Cancer: Results From a Phase Ib Study. Clin Breast Cancer 2021;21:539-51. [Crossref] [PubMed]
- Ferraro E, Drago JZ, Modi S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res 2021;23:84. [Crossref] [PubMed]
Cite this article as: Wang X, Li W, Yin Y, Tong Z, Zhang Q, Zheng H, Shao Z, Li H, Yang J, Feng J, Wu F, Lamour F, Restuccia E, Jiang Z. Primary results of ELAINA: a randomized, multicenter, open-label, phase III study of the efficacy and safety of trastuzumab emtansine vs. lapatinib plus capecitabine in Chinese patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy. Transl Breast Cancer Res 2023;4:3.



