
Efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib in patients with HR+HER2− metastatic breast cancer
Highlight box
Key findings
• Abemaciclib-based therapy showed superior efficacy to tucidinostat-based therapy in patients progressed on palbociclib.
What is known and what is new?
• Switching to another CDK4/6i, or histone deacetylase inhibitor tucidinostat are considerable treatment strategies for patients progressed on CDK4/6i in China.
• However, no clinical data has been reported on which of the two strategies is more effective. Our study showed that abemaciclib-based therapy significantly prolonged PFS compared with tucidinostat-based therapy in patients with HR+HER2− MBC who had progressed on palbociclib.
What is the implication, and what should change now?
• Our study suggests superiority of abemaciclib-based therapy over tucidinostat-based therapy in patients progressed on palbociclib, which may help clinicians make appropriate treatment recommendations and merits further assessment in larger and prospective trials.
Introduction
Hormone receptor-positive HER2-negative (HR+/HER2−) breast cancer accounts for approximately 65% of all metastatic breast cancer (MBC), which is the most common malignancy among women (1,2). For patients with HR+HER2− MBC, cyclin-dependent kinase 4 and 6 inhibitor (CDK4/6i) in combination with endocrine therapy (ET) have been recommended as the preferred treatment options according to guidelines (3-5). With the widespread application of CDK4/6i, there is an increasing number of cases progressed on CDK4/6i.
For post-CDK4/6i treatment, there is no standard of care according to international and domestic guidelines. Switching to another CDK4/6i, or targeted drugs with different mechanisms are considerable treatment options (3-5). Palbociclib is the first CDK4/6i approved for HR+HER2− MBC in China, followed by abemaciclib. Both palbociclib and abemaciclib have significantly prolonged the progression-free survival (PFS) in endocrine-sensitive and endocrine-resistant MBC in randomized controlled trials (6,7). As a highly selective CDK4/6 inhibitor, abemaciclib has shown its remarkable performance (8-12), including single-agent activity, reducing the risk of recurrence and significant improvement in overall survival. These above reasons led to application of abemaciclib as a second course of CDK4/6i-based therapy after the progression on palbociclib (13-15). Tucidinostat, an oral subtype-selective histone deacetylase inhibitor, was approved for patients with HR+HER2− MBC in China in 2019. In the ACE trial, tucidinostat plus exemestane improved PFS compared with placebo plus exemestane in patients with HR+HER2− MBC that progressed after prior ET (16). However, the efficacy of tucidinostat post-CDK4/6i is unclear.
With an increasing number of patients who have progressed on CDK4/6i, a second course of CDK4/6i and tucidinostat-based therapy are commonly used in clinical practice in China, and are recommended as considerable treatment options post-CDK4/6i according to Chinese Society of Clinical Oncology Breast Cancer guideline (CSCO BC guideline). Up to now, no clinical data has been reported on which of the two treatment strategies is more effective. In order to explore optimal treatment strategy post-CDK4/6i, using multicenter data from the database of CSCO BC, we performed a retrospective comparative cohort study to evaluate the efficacy and safety of abemaciclib-based therapy and tucidinostat-based therapy after progression on palbociclib. We present this article in accordance with the STROBE reporting checklist (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-9/rc).
Methods
Study design and participants
This is a retrospective comparative cohort study without intervention on treatment. We screened patients with HR+HER2− MBC who had received abemaciclib-based or tucidinostat-based therapy after progression on palbociclib between April 1st 2020 and June 30th 2022 from the CSCO BC database (CSCOBC RWS 2205). The data cutoff date was September 30th, 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Board of the Affiliated Hospital of Qingdao University (No. QYFYKYLL 221311920) and individual consent for this retrospective analysis was waived.
Main inclusion and exclusion criteria were as follows.
Inclusion criteria: (I) female, age ≥18 years; (II) pathologically diagnosed breast cancer with metastatic disease; (III) either primary or metastatic tumor that expressed HR positive and HER2 negative, HR positive was defined as the ratio of ER or PR positive cells detected by immunohistochemistry (IHC) ≥1%, HER2 negative was defined as IHC 0–1+ or 2+ and fluorescence in situ hybridization (FISH)/chromogenic in situ hybridization (CISH) non-amplified; (IV) patients have progressed on palbociclib; (V) patients have received abemaciclib-based therapy or tucidinostat-based therapy after progression on palbociclib.
Exclusion criteria: (I) incomplete medical information of palbociclib-based therapy and abemaciclib/tucidinostat-based therapy; (II) discontinuation of palbociclib due to non-disease progression, such as discontinuation because of adverse events and other non-medical factors.
Data collection
We collected data on patient demography, clinical and pathological status, treatment in the (neo)adjuvant and metastatic setting, palbociclib use, efficacy and safety evaluation of abemaciclib-based and tucidinostat-based therapies, multigene sequencing results before or during abemaciclib/tucidinostat-based therapy (including targeted sequencing of circulating tumor DNA and/or DNA detection in tumor tissue), which were derived from seven research centers’ inpatient and outpatient medical records. The patients were followed up by routine medical visits, or telephone calls with patients or their families.
Patients who received abemaciclib-based therapy composed abemaciclib group, and those who received tucidinostat-based therapy composed tucidinostat group. Patients who received both of the two treatments after progression on palbociclib were divided according to the treatment that patients received earlier.
The primary endpoint was PFS, defined as the time from the start of abemaciclib-based or tucidinostat-based treatment to the date of first disease progression [according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1] or death from any cause. The secondary endpoints were clinical benefit rate (CBR), safety and PFS according to PIK3CA gene type. CBR was defined as complete response (CR), partial response (PR), or stable disease (SD) for at least 6 months. Distant relapse-free interval (DRFI) was defined as the time from diagnosis to relapse at a distant site. Sequential use referred to abemaciclib/tucidinostat-based therapy was used immediately after palbociclib-based therapy. Non-sequential use referred to receiving one or several other treatments after palbociclib-based therapy and before Abemaciclib/tucidinostat-based therapy. Sensitive to palbociclib was defined as clinical benefit from prior palbociclib-based therapy, including CR, PR and SD ≥6 months. For each patient, the frequency and severity of adverse events and laboratory abnormalities [Common Terminology Criteria for Adverse Events (CTCAE) version 4.03] that occurred during the treatment course were recorded.
Statistical analysis
Disease characteristics, best overall response, frequency of breast cancer recurrent driver genomic alterations (17,18) and adverse events were summarized through descriptive analysis. Chi-square test was used to examine the association between patients’ baseline characteristics and treatment options. Kaplan-Meier method was applied to describe the distribution of PFS, Log-rank test was used to compare the survival curves of the two treatment groups and univariate Cox proportional hazards model was used to obtain the estimates of crude hazard ratio and its 95% CI.
Multivariate Cox model was applied to adjust potential confounders at baseline between the two treatment groups. Variables included in the final model were selected based on the consideration of both statistical significance and clinical importance. Univariate Cox model was first used to evaluate the crude association between predictor variable and treatment outcome, variables which were statistically significant in the univariate model and were considered clinically important based on prior information were included in the final model. As a sensitivity analysis, propensity score matching (PSM) was also performed to adjust potential confounders. Population was sampled and balanced using propensity score which was calculated based on palbociclib-treated PFS (<12 or ≥12 months), number of previous lines of ET for MBC (1–2 or ≥3) and whether sequential use after palbociclib. The matching approach was 1:1 nearest neighbor with caliber of 20% and survival analysis method was applied among this PSM population to estimate the effect of treatment.
Moreover, subgroup analysis was also performed to evaluate the effect of treatment according to age, HR status, sensitivity to prior palbociclib, sequential or non-sequential use after palbociclib, visceral disease, number of metastatic organs and prior lines of ET for MBC. Multivariate Cox regression was performed within each stratum of the subgroups to estimate the adjusted treatment effect. Variables in the final models included all variables in the whole study population model.
All statistical analyses were done in SAS (version 9.4, Cary, NC, USA). All P values were two-sided, with 0.05 as the level of statistical significance.
Results
Patient characteristics
A total of 149 patients were identified, of whom 73 patients received abemaciclib plus ET, and 76 patients received tucidinostat plus ET. The characteristics of patients in the two groups were shown in Table 1. The majority of patients had visceral disease (124/149, 83.2%) and ≥3 metastatic organs (76/149, 51.0%), one third (48/149, 32.2%) had previously been treated ≥3 lines of ET in MBC setting. The proportion of patients received sequential treatment was higher in abemaciclib group (36/73, 49.3%) than that in tucidinostat group (23/76, 30.3%). There were no statistically significant differences in other baseline characteristics between the two groups.
Table 1
| Characteristics | ET + abemaciclib (n=73) | ET + tucidinostat (n=76) | P value |
|---|---|---|---|
| Age, years, median [range] | 51.8 [30–78] | 53.2 [31–76] | 0.42 |
| Hormone receptor status, n (%) | 0.20 | ||
| ER+/PR+ | 60 (82.2) | 68 (89.5) | |
| ER+/PR− | 13 (17.8) | 8 (10.5) | |
| Distant relapse-free interval, n (%) | 0.50 | ||
| De novo stage IV | 10 (13.7) | 13 (17.1) | |
| <24 months | 11 (15.1) | 7 (9.2) | |
| ≥24 months | 52 (71.2) | 56 (73.7) | |
| Palbociclib treatment course | |||
| Sensitive to palbociclib, n (%) | 0.10 | ||
| Yes | 56 (76.7) | 49 (64.5) | |
| No | 17 (23.3) | 27 (35.5) | |
| Abemaciclib/tucidinostat treatment course | |||
| Sequential use after palbociclib, n (%) | 0.02 | ||
| Yes | 36 (49.3) | 23 (30.3) | |
| No | 37 (50.7) | 53 (69.7) | |
| Previous lines of ET for MBC, median [range] | 2 [1–7] | 2 [1–6] | 0.60 |
| 1–2, n (%) | 48 (65.8) | 53 (69.7) | |
| ≥3, n (%) | 25 (34.2) | 23 (30.3) | |
| Endocrine partner*, n (%) | 0.71 | ||
| Aromatase inhibitor | 30 (41.1) | 39 (51.3) | |
| Fulvestrant | 29 (39.7) | 20 (26.3) | |
| Other† | 13 (17.8) | 17 (22.4) | |
| Visceral disease, n (%) | 0.10 | ||
| Yes | 57 (78.1) | 67 (88.2) | |
| No | 16 (21.9) | 9 (11.8) | |
| Number of metastatic organs, n (%) | 0.81 | ||
| 1 | 15 (20.5) | 12 (15.8) | |
| 2 | 21 (28.8) | 25 (32.9) | |
| ≥3 | 37 (50.7) | 39 (51.3) | |
*, one case missing in abemaciclib group; †, other included tamoxifen, toremifene, and progesterone. ET, endocrine therapy; ER, estrogen receptor; PR, progesterone receptor; MBC, metastatic breast cancer.
The most commonly used ET partner was aromatase inhibitor (69/149, 46.3%), followed by fulvestrant (49/149, 32.9%). None received abemaciclib or tucidinostat as a single agent. In contrast with the palbociclib-based setting, 87.9% (131/149) of the total patients transitioned to novel ET partner in the abemaciclib-based or tucidinostat-based setting (Figure S1). Dosage and dose adjustment information was shown in Table S1.
By the data cutoff date (September 30th, 2022), 123 events of disease progression or death had occurred, with 53 cases (53/73, 72.6%) in abemaciclib group and 70 cases (70/76, 92.1%) in tucidinostat group, respectively. Fifteen patients (15/73, 20.5%) in abemaciclib group and 4 (4/66, 5.3%) in tucidinostat group were still receiving treatment. Five patients in abemaciclib group and 2 patients in tucidinostat group were lost of follow-up. Overall, the median follow-up time was 9.0 months (IQR, 5.5–13.0 months).
Efficacy analysis of abemaciclib-based and tucidinostat-based therapy
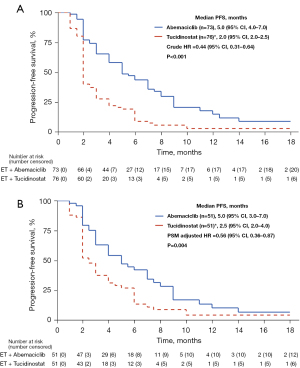
In terms of efficacy analysis, there was no patient with CR or PR in tucidinostat group. More patients in abemaciclib group experienced clinical benefit than those in tucidinostat group (38.4% vs. 17.1%, P=0.004) (Table 2). In total population, after adjusting for potential confounders, patients receiving abemaciclib-based therapy and sequential use of abemaciclib/tucidinostat-based therapy after progression on palbociclib were associated with better clinical outcome in terms of PFS (Table 3). The median PFS was 5.0 months (95% CI: 4.0–7.0) in abemaciclib group and 2.0 months (95% CI: 2.0–2.5) in tucidinostat group [crude hazard ratio =0.44 (95% CI: 0.31–0.64); P<0.001] (Figure 1A). In propensity score matched population (51 cases in each group), statistical difference still existed between PFS in abemaciclib group and that in tucidinostat group [5.0 vs. 2.5 months, PSM adjusted hazard ratio =0.56 (95% CI: 0.36–0.87); P=0.004] (Figure 1B).
Table 2
| Treatment response | ET + abemaciclib (n=73) | ET + tucidinostat (n=76) | P value |
|---|---|---|---|
| Best overall response | |||
| CR | 0 | 0 | |
| PR | 3 (4.1%) | 0 | |
| SD | 55 (75.3%) | 31 (40.8%) | |
| PD | 15 (20.5%) | 45 (59.2%) | |
| Objective response (CR + PR) | 3 (4.1%) | 0 | |
| Clinical benefit (CR + PR + SD ≥6 months)† | 28 (38.4%) | 13 (17.1%) | 0.004 |
†, number of patients SD ≥6 months in abemaciclib group was 25; number of patients SD ≥6 months in tucidinostat group was 13. ET, endocrine therapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Table 3
| Factors | Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Group | |||||||
| ET + tucidinostat (ref) | |||||||
| ET + abemaciclib | 0.44 | 0.31–0.64 | <0.01 | 0.46 | 0.31–0.68 | <0.01 | |
| Age | |||||||
| ≥60 years (ref) | |||||||
| <60 years | 1.11 | 0.72–1.71 | 0.64 | 1.03 | 0.65–1.64 | 0.89 | |
| Hormone receptor status | |||||||
| ER+/PR− (ref) | |||||||
| ER+/PR+ | 0.99 | 0.59–1.65 | 0.96 | 0.93 | 0.54–1.63 | 0.81 | |
| Distant relapse-free interval | |||||||
| ≥24 months (ref) | |||||||
| <24 months | 0.96 | 0.56–1.67 | 0.90 | 1.42 | 0.80–2.54 | 0.23 | |
| De novo stage IV | 1.08 | 0.65–1.80 | 0.77 | 0.90 | 0.53–1.56 | 0.72 | |
| Sensitive to previous palbociclib | |||||||
| No (ref) | |||||||
| Yes | 0.72 | 0.49–1.07 | 0.10 | 1.00 | 0.63–1.60 | 0.99 | |
| PFS of palbociclib for MBC | |||||||
| <12 months (ref) | |||||||
| ≥12 months | 0.63 | 0.44–0.92 | 0.02 | 0.69 | 0.44–1.08 | 0.10 | |
| Sequential use after palbociclib | |||||||
| No (ref) | |||||||
| Yes | 0.55 | 0.38–0.80 | <0.01 | 0.58 | 0.39–0.86 | <0.01 | |
| Visceral disease | |||||||
| No (ref) | |||||||
| Yes | 2.12 | 1.25–3.62 | <0.01 | 1.67 | 0.94–2.97 | 0.08 | |
| Number of metastatic organs | |||||||
| ≥3 (ref) | |||||||
| 1–2 | 0.67 | 0.47–0.96 | 0.03 | 0.71 | 0.48–1.04 | 0.08 | |
| Prior lines of ET for MBC | |||||||
| ≥3 (ref) | |||||||
| 1–2 | 1.04 | 0.71–1.51 | 0.84 | 1.16 | 0.76–1.77 | 0.49 | |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ET, endocrine therapy; ER, estrogen receptor; PR, progesterone receptor; MBC, metastatic breast cancer.
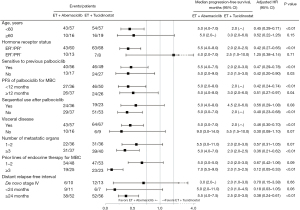
Subgroups analysis was shown in Figure 2. The superiority of PFS in abemaciclib group was consistent across most subgroups, especially among patients with refractory factors, such as visceral disease (hazard ratio: 0.46, P<0.01), number of metastatic organs ≥3 (hazard ratio: 0.36, P<0.01) and prior lines of ET for MBC ≥3 (hazard ratio: 0.12, P<0.01). For patients receiving abemaciclib-based therapy, there was no significant difference in PFS between patients sensitive and non-sensitive to palbociclib-based therapy (5.5 vs. 5.0 months, P=0.3127) (Figure S2A).
Safety information
Hematological toxicities were common in both the abemaciclib and tucidinostat groups, and the most common grade 3 or 4 hematological adverse events in either group was neutropenia (Table 4). Anemia and thrombocytopenia occurred in more than 30% of patients in tucidinostat group, while thrombocytopenia occurred in a lower proportion of patients in abemaciclib group, only 9.5%. The most common non-hematological toxicity was diarrhea (27.4%) in abemaciclib group and increased aspartate aminotransferase (AST) (26.3%) in tucidinostat group. Other common non-hematological toxicity occurred in tucidinostat group included nausea (25.0%), vomiting (11.8%) and hypokalemia (13.2%). The incidence of grade 3–4 non-hematological adverse reactions in both groups was lower than 2.0%.
Table 4
| Adverse events | ET + abemaciclib (n=73) | ET + tucidinostat (n=76) | |||
|---|---|---|---|---|---|
| Grade 1–2, n (%) | Grade 3–4, n (%) | Grade 1–2, n (%) | Grade 3–4, n (%) | ||
| Any | 46 (63.0) | 11 (15.1) | 58 (76.3) | 9 (11.8) | |
| Leukopenia | 19 (26.0) | 3 (4.1) | 22 (28.9) | 2 (2.6) | |
| Neutropenia | 13 (17.8) | 4 (5.5) | 19 (25.0) | 7 (9.2) | |
| Anemia | 16 (21.9) | 1 (1.4) | 24 (31.6) | 0 | |
| Thrombocytopenia | 5 (6.8) | 2 (2.7) | 26 (34.2) | 1 (1.3) | |
| ALT increased | 9 (12.3) | 1 (1.4) | 13 (17.1) | 0 | |
| AST increased | 12 (16.4) | 1 (1.4) | 20 (26.3) | 0 | |
| Diarrhea | 19 (26.0) | 1 (1.4) | 4 (5.3) | 0 | |
| Fatigue | 9 (12.3) | 0 | 9 (11.8) | 0 | |
| Abdominal pain | 3 (4.1) | 0 | 2 (2.6) | 0 | |
| Interstitial pneumonia | 1 (1.4) | 0 | 2 (2.6) | 0 | |
| Infectious pneumonia | 1 (1.4) | 0 | 0 | 0 | |
| Hyperbilirubinemia | 3 (4.1) | 0 | 3 (3.9) | 0 | |
| Nausea | 3 (4.1) | 0 | 19 (25.0) | 0 | |
| Vomiting | 0 | 0 | 9 (11.8) | 0 | |
| Hypokalemia | 2 (2.7) | 0 | 10 (13.2) | 0 | |
| Mucositis | 1 (1.4) | 0 | 1 (1.3) | 0 | |
| Abdominal distention | 0 | 0 | 4 (5.3) | 0 | |
| Hyperglycemia | 0 | 0 | 4 (5.3) | 1 (1.3) | |
| Edema | 0 | 0 | 3 (3.9) | 0 | |
ET, endocrine therapy; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Dose reduction due to adverse events occurred in 6 patients (6/73, 8.2%) in abemaciclib group and 8 patients (8/76, 10.5%) in tucidinostat group, mainly because of diarrhea and hematological toxicity in abemaciclib group, thrombocytopenia, nausea and vomiting in tucidinostat group (Table S1). One patient in tucidinostat group discontinued treatment because of simultaneous occurrence of hematological toxicity, grade 3 hyperglycemia and stomach pain.
Frequency of breast cancer recurrent driver genomic alterations and PFS according to PIK3CA type
A total of 43 patients underwent multigene sequencing before or during abemaciclib/tucidinostat-based therapy by next generation sequencing (NGS). Frequency of breast cancer recurrent driver genomic alterations (17,18) (alteration frequency greater than 4.0%) was shown in Figure 3A and Table S2. PIK3CA mutations occurred in 44.20% (19/43) of patients and ESR1 mutations occurred in 25.6% (11/43) of patients. In patients with PIK3CA-mutant, there was no significant difference in PFS between abemaciclib group and tucidinostat group (Figure 3B). In patients with PIK3CA wild-type, median PFS was 6.0 months in abemaciclib group and 2.0 months in tucidinostat group, but the difference was not significant (Figure 3C). For patients receiving abemaciclib, median PFS in patients with PIK3CA wild-type was longer than that in patients with PIK3CA-mutant (6.0 vs. 3.0 months, P=0.0498) (Figure S2B).

Discussion
Our study focused on comparison analysis of switching to another CDK4/6i and targeted drug with different mechanism, which may drive decision making in treatment strategies post-CDK4/6i. In this multicenter real-world study, we assessed efficacy of abemaciclib-based therapy versus tucidinostat-based therapy in patients progressed on palbociclib. We found that the combination of abemaciclib and ET improved CBR and prolonged PFS compared with the combination of tucidinostat and ET.
Second course of CDK4/6i-based therapy is under highly active clinical trial investigation, and published studies include single arm retrospective study, prospective MAINTAIN study, and TRINITI-1 study (12,19,20). The median PFS of abemaciclib group in our study was 5.0 months, which is similar to that of switching to another CDK4/6i in retrospective and prospective studies (about 5.3 months), confirming the benefit of second course of CDK4/6i-based therapy.
As a HDAC inhibitor, tucidinostat plus exemestane improved PFS compared with placebo plus exemestane in ACE study (16). However, in our study the combination of tucidinostat and ET did not show any objective response in patients progressed on palbociclib, and most patients experienced disease progression at the first efficacy evaluation. By comparison, abemaciclib group was associated with a CBR of 38.4%. In terms of baseline characteristics, the proportion of patients non-sensitive to prior palbociclib was slightly higher in tucidinostat group than that in abemaciclib group, which may partly explain the rapid disease progression in tucidinostat group. Although most patients transitioned to novel ET partner in the abemaciclib/tucidinostat-based setting, tumor could hardly response to ET due to development of ET-resistance. Our data indicated that the addition of tucidinostat to ET did not show active anti-tumor performance on tumor progressed during CDK4/6i and ET.
Although palbociclib and abemaciclib have similar pharmacological effects, abemaciclib has unique properties, including increased selectivity for CDK4 over CDK6, inhibiting CDK4/6 at low nanomolar concentrations, continuous administration, which have led to remarkable clinical performance in early-stage breast cancer and MBC (21-23). In such a refractory population, treatment options are typically limited. The median PFS of abemaciclib group in our study was 5.0 months, notably similar to PFS of abemaciclib monotherapy in heavily pretreated HR+HER2− MBC in MONARCH-1 study (21-23). It is likely that single-agent activity of abemaciclib makes it possible that its anti-tumor performance does not depend on endocrine pathways, leading to considerable CBR of abemaciclib in case of endocrine-resistance. Another possible reason for superiority of abemaciclib group may be the simultaneous inhibition of estrogen receptor and CDK4/6-cyclinD-Rb signaling pathway which are important main drivers of cancer cell growth and survival in HR+/HER2− tumors (24-26).
The results of univariable and multivariable Cox regression analysis showed that abemaciclib-based therapy and sequential use of abemaciclib/tucidinostat-based therapy after progression on palbociclib were associated with better clinical outcome in terms of PFS. In subgroup analysis, superiority of PFS in abemaciclib group was consistent across most subgroups, especially among patients with refractory factors. Besides, sensitivity to prior palbociclib, sequential or non-sequential treatment after palbociclib seem to have little effect on the benefit of abemaciclib-based therapy. However, the sample size of this study is relatively small, which requires further research. Actually, most of patients with non-sequential use of CDK4/6i had received chemotherapy as subsequent-line therapy after progression on palbociclib. Patients could benefit from both sequential and non-sequential use of abemaciclib-based therapy after palbociclib, indicating that ether continuous or intermittent co-inhibition of estrogen receptor and CDK4/6-cyclinD-Rb signaling pathway may be potentially effective for HR+HER2− MBC. For tucidinostat-based therapy after palbociclib, sequential use may be preferable.
Given widespread application of CDK4/6i, it is urgent to explore CDK4/6i resistance mechanism and identify beneficial factors for a second course of CDK4/6i-based therapy. The study on drug resistance mechanism in PALOMA-3 trial showed that ESR1 and PIK3CA were the most commonly mutated genes in a CDK4/6i- and endocrine-resistant patient population (27). It is known that ESR1 mutations are an important mechanism of resistance to aromatase inhibitors (28,29), and PIK3CA mutations are important founding variants in ER-positive primary breast cancers (18,30). In our study, PIK3CA mutations occurred in 44.20% of patients and ESR1 mutations occurred in 25.6% of patients who had undergone multigene sequencing. Not surprisingly, PIK3CA-mutant was associated with worse clinical outcome in terms of PFS in both abemaciclib and tucidinostat groups; while in patients with PIK3CA wild-type, there was an absolute difference of 4.0 months in PFS between abemaciclib and tucidinostat group. For patients receiving abemaciclib, PIK3CA-mutant showed a negative effect on PFS. According to NCCN and ABC5 guidelines, PI3K inhibitor alpelisib is recommended to be used after CDK4/6i for patients with PIK3CA-mutated MBC (3,4). These data indicated that metastatic tumor biopsy for repeat biomarker status and genomic sequencing should be strongly considered while progressed on CDK4/6i.
This study has some limitations, such as the retrospective nature of the data collection and physician bias in the selection of treatment strategies. Besides, adverse events incidences in abemaciclib group and tucidinostat group seem lower than those in randomized controlled trials due to the nature of retrospective data collection. On the whole, in the absence of head-to-head randomized controlled trial, our study provide important data reference for patients progressed on palbociclib.
Conclusions
In conclusion, this multicenter study demonstrated that abemaciclib-based therapy was superior to tucidinostat-based therapy in terms of CBR and PFS in patients progressed on palbociclib. Even in patients with non-sensitive to prior palbociclib, heavy tumor load and multi-lines of ET, patients tend to benefit from abemaciclib rather than from tucidinostat. PIK3CA-mutant showed a negative effect on PFS, which indicated that multigene sequencing should be strongly considered when disease progressed during CDK4/6i. These findings may help clinicians make appropriate treatment option after progression on CDK4/6i.
Acknowledgments
We thank Professor Linlin Jiang from the Graduate School of the PLA General Hospital for editing the English text of this manuscript. The authors thank all of the patients and their families, as well as physicians in the seven research centers.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-9/rc
Data Sharing Statement: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-9/dss
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-9/coif). YW is a full-time employee of Medical Department, Medpion (Beijing) Medical Technology Co., Ltd., Beijing and LZ is a full-time employee of Genecast (Beijing) Biotechnology Co., Ltd. Yongmei Yin, SW, and TS serve as unpaid editorial board members of Translational Breast Cancer Research from March 2022 to February 2024. JL serves as the unpaid managing editor of Translational Breast Cancer Research from November 2019 to October 2024. ZJ serves as the Editor-in-Chief of Translational Breast Cancer Research. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Board of the Affiliated Hospital of Qingdao University (No. QYFYKYLL 221311920) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069-103. [Crossref] [PubMed]
- Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat 2013;141:507-14. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology—Breast Cancer Version 1.2022. Accessed Nov 24, 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49. [Crossref] [PubMed]
- Jiang Z, Li J, Chen J, et al. Chinese Society of Clinical Oncology (CSCO) Breast Cancer Guidelines 2022. Transl Breast Cancer Res 2022;3:13. [Crossref]
- Zhang QY, Sun T, Yin YM, et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2- advanced breast cancer: the multinational randomized phase III study. Ther Adv Med Oncol 2020;12:1758835920963925. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(-) Metastatic Breast Cancer. Clin Cancer Res 2017;23:5218-24. [Crossref] [PubMed]
- Wander SA, O'Brien N, Litchfield LM, et al. Targeting CDK4 and 6 in Cancer Therapy: Emerging Preclinical Insights Related to Abemaciclib. Oncologist 2022;27:811-21. [Crossref] [PubMed]
- Tolaney SM, Sahebjam S, Le Rhun E, et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor-Positive Breast Cancer. Clin Cancer Res 2020;26:5310-9. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol 2020;6:116-24. [Crossref] [PubMed]
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020;38:3987-98. [Crossref] [PubMed]
- Wander SA, Han HS, Zangardi ML, et al. Clinical Outcomes With Abemaciclib After Prior CDK4/6 Inhibitor Progression in Breast Cancer: A Multicenter Experience. J Natl Compr Canc Netw 2021;1-8. [Crossref] [PubMed]
- Li Y, Li W, Gong C, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol 2021;13:17588359211022890. [Crossref] [PubMed]
- Martin JM, Handorf EA, Montero AJ, et al. Systemic Therapies Following Progression on First-line CDK4/6-inhibitor Treatment: Analysis of Real-world Data. Oncologist 2022;27:441-6. [Crossref] [PubMed]
- Jiang Z, Li W, Hu X, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:806-15. [Crossref] [PubMed]
- Condorelli R, Mosele F, Verret B, et al. Genomic alterations in breast cancer: level of evidence for actionability according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol 2019;30:365-73. [Crossref] [PubMed]
- Lefebvre C, Bachelot T, Filleron T, et al. Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis. PLoS Med 2016;13:e1002201. [Crossref] [PubMed]
- Kalinsky K, Accordino MK, Chiuzan C, et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J Clin Oncol 2022;40:LBA1004. [Crossref]
- Bardia A, Hurvitz SA, DeMichele A, et al. Phase I/II Trial of Exemestane, Ribociclib, and Everolimus in Women with HR(+)/HER2(-) Advanced Breast Cancer after Progression on CDK4/6 Inhibitors (TRINITI-1). Clin Cancer Res 2021;27:4177-85. [Crossref] [PubMed]
- Hafner M, Mills CE, Subramanian K, et al. Multiomics Profiling Establishes the Polypharmacology of FDA-Approved CDK4/6 Inhibitors and the Potential for Differential Clinical Activity. Cell Chem Biol 2019;26:1067-1080.e8. [Crossref] [PubMed]
- O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 2016;13:417-30. [Crossref] [PubMed]
- Braal CL, Jongbloed EM, Wilting SM, et al. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021;81:317-31. [Crossref] [PubMed]
- Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov 2016;6:353-67. [Crossref] [PubMed]
- Pearce ST, Jordan VC. The biological role of estrogen receptors alpha and beta in cancer. Crit Rev Oncol Hematol 2004;50:3-22. [Crossref] [PubMed]
- Ozyurt R, Ozpolat B. Molecular Mechanisms of Anti-Estrogen Therapy Resistance and Novel Targeted Therapies. Cancers (Basel) 2022;14:5206. [Crossref] [PubMed]
- O'Leary B, Cutts RJ, Liu Y, et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov 2018;8:1390-403. [Crossref] [PubMed]
- Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015;12:573-83. [Crossref] [PubMed]
- Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 2013;45:1439-45. [Crossref] [PubMed]
- Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70. [Crossref] [PubMed]
Cite this article as: Yuan Y, Zhang S, Wang T, Wang B, Wang S, Shi J, Sun T, Yin Y, Ouyang Q, Li J, Wen Y, Zhang L, Jiang Z. Efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib in patients with HR+HER2− metastatic breast cancer. Transl Breast Cancer Res 2023;4:10.


