Types and progress of clinical trial design for breast cancer: a narrative review
Introduction
Breast cancer, being one of the most common forms of cancer worldwide, poses a significant global health burden (1,2). As per the Global Cancer Observatory 2022 estimates (3), breast cancer accounts for 11.7% of all new cancer cases, underscoring its widespread prevalence. It is a heterogeneous disease, with variations in molecular subtypes, stages at diagnosis, and responses to therapy, leading to diverse patient outcomes (4).
Clinical trials play a pivotal role in advancing breast cancer treatment (5). They serve as the backbone for the development and approval of new therapies, offering more options and hope for patients. These rigorously designed studies assess the safety, efficacy, and optimal use of new interventions, contributing significantly to improving survival rates and quality of life in breast cancer patients.
The progression of clinical trial design is continuously changing the way we evaluate new therapeutic methods. Traditional clinical trial designs, such as phase I–IV trials, have been in place for decades. However, with scientific and technological advancements, a surge of innovative clinical trial designs has emerged, including seamless trials, master protocols (encompassing basket trials, umbrella trials, and platform trials), enrichment designs, and marker stratified designs. Although numerous reviews exist regarding these innovative designs (5-9), many focus predominantly on the theoretical and statistical methods, with less emphasis on their specific applications and advancements within a particular disease area like breast cancer. Therefore, this review aims to summarize and review the application and progression of these innovative clinical trial designs in the field of breast cancer research, hoping to provide useful guidance for researchers engaged in breast cancer and related fields. We present this article in accordance with the Narrative Review reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-22/rc).
Methods
In summary, we conducted a comprehensive search of major databases to identify relevant studies on innovations in breast cancer clinical trial design published over the past 20 years. Rigorous criteria were applied to screen and select articles for inclusion. The search strategy is summarized in Table 1. Relevant guidelines were also reviewed to supplement the literature review. This thorough approach allowed us to compile a representative collection of studies to inform the discussion in this review.
Table 1
| Items | Specification |
|---|---|
| Date of search | 1-May-2023 |
| Databases and other sources searched | PubMed, Embase, Cochrane Library |
| Search terms used | “Breast cancer”, “clinical trial”, “seamless”, “master protocol”, “umbrella”, “basket”, “platform”, and “precision medicine” |
| Timeframe | Last 20 years |
| Inclusion and exclusion criteria | Inclusion criteria: (I) articles focused on breast cancer; (II) articles discussing innovative clinical trial designs; (III) English language; (IV) humans; (V) last 20 years. Exclusion criteria: (I) non-English articles; (II) reviews, editorials, letters, conference abstracts; (III) non-human studies; (IV) articles not focused on breast cancer or clinical trial design innovations |
| Selection process | The screening and selection were conducted independently by two reviewers. Any disagreements were resolved by discussion and consensus |
| Any additional considerations, if applicable | The search was supplemented by scanning reference lists of relevant articles and reviewing relevant guidelines |
The evolution of clinical trial design in breast cancer
Overview of traditional clinical trial design in breast cancer
Clinical trials involve systematic investigation using human subjects (patients or healthy volunteers) to discover or validate the clinical, pharmacological, and other pharmacodynamic effects of a particular drug for a specific disease. They aim to evaluate the drug’s efficacy and safety (9). Traditionally, clinical trials are divided into phases I–III before drug approval and phase IV after drug marketing.
The essence of phases I–III (10) is to explore the tolerance, pharmacokinetics, dosages, administration schedules, and therapeutic effectiveness of a drug. The specific challenges posed by breast cancer trials require particular attention. Notably, patient baseline characteristics, comorbidities, unique molecular tumor features, and significant variability in the microenvironment lead to imbalances that can impact the results, even with well-planned trials.
Phase IV trials (11), conducted post-marketing, aim to evaluate the benefit-risk relationship in general or specific breast cancer populations, with a focus on dosage optimization.
In general, these clinical studies had an overall success rate of about 38% (6,7), with blinded randomized studies being the gold standard as they minimize bias. However, given the complexities and inherent variability, it’s paramount to devise and implement cutting-edge clinical trial designs.
Innovations in clinical trial design
Both in China and overseas, great changes have recently been made to the research and development model for anti-breast cancer medications and strategies. The first change reflects the speed of research progress. The “seamless design” approach has increasingly taken the place of the conventional three-stage drug research and development model. The phase gap among phase I, II and III trials was intended to be eliminated by the seamless phase I/II or phase II/III design. The second change is reflected in the breadth of research progress. The master plan study has been utilized more frequently, and the basket study and umbrella study are the more representative studies. The former aims to evaluate the therapeutic effects of a single drug in treating various disease types with the same biological characteristics and explores “treatment for different diseases”, the latter aims to evaluate the therapeutic effects of a number of therapies/regimens for patients with a single breast cancer molecular classification but various biomarker types and explore the “treatment for different diseases”. The last change is reflected in the precision of the research progress. The key goals of research are to identify the most suitable beneficiary population and to handle test medications in a tailored manner. This is because more and more clinical trial decisions are being influenced by biomarkers due to the current era of precision tumor. As a result, various clinical research designs for studying breast cancer, like enrichment designs and marker stratification designs, continually appear.
The acceleration in the speed of design: the emergence of seamless trials
Seamless trials design (12) is a revolutionary approach to clinical trials that enables the integration of different phases of trials into a continuous process, thus aiming to improve efficiency, reduce costs, and expedite drug development. This approach can be utilized across various stages, not only limited to phase II/III but also applicable to phase I/II trials. By eliminating the downtime between phases, the design can hasten the assessment of therapies or treatments, particularly crucial when conventional confirmatory phase III trials may be time-consuming due to the requirement for survival benefit data.
For example, a seamless phase II/III design that attempts to eliminate the blank period between phase II and III trials may be either an operationally seamless design that excludes phase II subjects from the primary analysis or an extrapolated seamless design that includes phase II subjects in the primary analysis. The former does not require multiplicity adjustments to the control of class I errors, while the latter may require corresponding adjustments based on the adaptive nature and hypothesis testing strategy.
Key considerations for seamless trials design
While the seamless trials design holds potential for boosting research efficiency and reducing development time, it also presents unique challenges, particularly in terms of ethical risk control. The accelerated trial progression may expose more patients to therapies with unknown toxicity and limited potential benefit, necessitating rigorous safety measures. The Food and Drug Administration (FDA) guidelines recommend monitoring and reporting safety issues, establishing an Independent Science Advisory Committee (ISAC) or an Independent Data Monitoring Committee (IDMC), maintaining regular communication with the Institutional Review Board (IRB), and providing regular updates of informed consent forms (13). As the field of drug development continues to flourish, the strategic use of seamless trials design to develop effective, safe, and controllable therapies in a more efficient manner is a shared goal and challenge among regulators, pharmaceutical companies, and researchers.
(I) Seamless trials design for breast cancer
This study (NCT0328056) (14,15) utilizes a two-stage design to evaluate the efficacy and safety of multiple immunotherapy combination regimens in patients with advanced HR-positive, HER2-negative breast cancer. In stage 1, patients are randomized to a control or an atezolizumab-containing doublet or triplet therapy. Based on efficacy and safety results, new combination treatments may be added in stage 2. The study emphasizes progression-free survival assessment and represents the potential for immunotherapy combinations in this patient population.
The improvement in the breadth of design: the advent of master protocols
In our previous section, we addressed the innovative strategy of seamless trial design, an approach that, while powerful and increasingly popular, is not as novel or unique as master protocols. It’s important to note that the seamless design is not restricted to certain stages; it can be applied across the spectrum of clinical trial phases, including phase I/II and phase II/III. Nonetheless, any trial, including those with master protocols, could theoretically be run using a seamless design.
While seamless trials offer a strategic advantage in accelerating the clinical development process, we highlight in this review the transformative impact and unique benefits of master protocol designs. This design allows multiple potential therapies to be tested concurrently within the same overall trial structure, which significantly enhances the efficiency and potential reach of clinical research. In the following sections, we will elaborate more on this promising design and discuss how it is changing the landscape of clinical trial designs, particularly in the context of breast cancer research.
Master protocols constitute an overarching structure that incorporates several sub-studies, each investigating different therapies, populations, or both, based on certain eligibility criteria. Three main types of master protocols exist: basket, umbrella, and platform trials (8) (Table 2).
Table 2
| Design type | Objective |
|---|---|
| Basket trial | To study one therapy across multiple diseases or disease subtypes possessing a common biomarker |
| Umbrella trial | To study multiple therapies within one disease based on molecular profiling |
| Platform design | To study multiple targeted therapies in the context of a single disease in a perpetual manner, with therapies allowed to enter or leave the platform on the basis of a decision algorithm |
Master protocol
A master protocol (8,16,17) is a unifying study design that includes multiple subgroups and sub-studies, with patients having same or different diseases and that employ one or multiple therapies to treat it. Initially designed for oncology, master protocol trials are intended to simultaneously evaluate more than one investigational drug and/or more than one disease type within the same overall trial structure. The ability to use a single infrastructure, trial design, and protocol to simultaneously evaluate multiple therapies and/or disease populations in multiple sub-studies speeds up drug development and makes it more efficient.
The key advantage of the master protocol design is the concept of “sharing” (18). By “sharing”, we mean pooling resources, data, and infrastructure across different sub-studies within the protocol. This approach can streamline processes, reduce duplication of effort, and improve the efficiency of trials. Furthermore, it allows for a consistent methodology to be applied across studies, improving comparability of results. Therefore, the concept of “sharing” in master protocols can lead to significant improvements in the speed and efficiency of drug development.
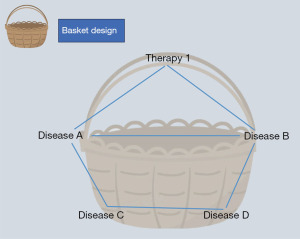
Basket design
Basket design is to study one therapy across multiple diseases or disease subtypes possessing a common biomarker (8). The goal of a basket trial is to evaluate the clinical efficacy of a targeted therapy (the ‘basket’) across various types of diseases that share this biomarker.
To illustrate, different cancers that carry the same target gene mutation would be ‘put into the same basket’ for investigation (Figure 1). For instance, in breast cancer trials, patients with HER2-positive cancer might be grouped into the same basket to examine the effectiveness of a specific targeted therapy, such as Herceptin (19).
(I) Basket design for breast cancer
Basket design in clinical trials has shown considerable promise, particularly in the study of breast cancer. This innovative approach allows the investigation of the effects of novel treatments across multiple tumor types, including those harboring rare mutations. By focusing on genetic alterations rather than organ-based tumor classification, basket trials present an opportunity to accelerate the development of effective treatments, particularly in cases of breast cancer with specific genetic alterations.
The value of this approach has been illustrated by several studies. One such example is the IMMU-132-01 study (NCT01631552), an open-label basket trial that evaluated the safety and efficacy of Sacituzumab Govitecan-hziy across multiple solid tumors, including metastatic triple-negative breast cancer (TNBC). The study demonstrated promising results in the treatment of previously treated metastatic TNBC (20), thereby demonstrating the potential of the basket trial design in extending our understanding of drug effects across various cancer types.
The B-AMAZE study (NCT03330405) (21), another example, applied a basket design for a phase I trial of larotrectinib in 10 advanced solid tumors with neurotrophic tyrosine receptor kinase (NTRK) fusions. This trial showed notable activity in advanced breast cancer, underscoring the potential of this design in guiding targeted therapy decisions based on the genomic profiles of tumors.
Finally, the TAPUR study (NCT02693535) (22) provides another example of a basket platform trial that assesses the efficacy of multiple targeted therapies across various cancer types, including breast cancer. This trial serves to illustrate how basket designs can be used to evaluate a variety of therapies in a single trial, thereby enhancing research efficiency and providing a feasible approach for studying new therapies across diverse cancer types that share a common biomarker.
In essence, these studies highlight the potential of basket designs in advancing our understanding of breast cancer treatment and, importantly, in potentially guiding the development of future targeted therapies.
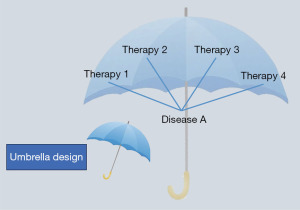
Umbrella design
Umbrella design is to study multiple therapies within one disease based on molecular profiling (8). Another name for this design is the “Umbrella Trial”, which refers to holding up a large umbrella to group lung cancer patients who have different driving genes, such as KRAS, EGFR, and ALK, under one large umbrella. The purpose of this large umbrella is to simultaneously complete different target detections and distribute various precise target therapies in accordance with various target genes (Figure 2) (8).
(I) Umbrella design for breast cancer
Advancements in the design of clinical trials for breast cancer have made substantial contributions to our understanding and management of this complex disease. In particular, umbrella trials, which evaluate multiple treatment options within a single disease population based on molecular subtypes, have heralded a new era of precision medicine.
In March 2023, a milestone study called “FUTURE” was published in the journal Cell Research by the team led by Professor Zhi-Ming Shao (23). This is the first umbrella phase II clinical trial in China focusing on metastatic recurrent TNBC. This trial stratified patients into seven treatment groups based on molecular classification, with each group receiving a different personalized treatment scheme. This trial marks a significant milestone in precision medicine, providing a model for designing personalized treatment plans based on molecular subtypes.
Another promising trial, NeoTRIP (NCT02889874) (24), is a phase I/II umbrella clinical trial that studies the effects of combined use of leukemia inhibitory factor (LIF) inhibitor E6201, paclitaxel, and carboplatin in newly diagnosed stage I–III TNBC patients. By evaluating the efficacy and safety of E6201 in a highly heterogeneous TNBC population, this study underscores the importance of umbrella design in effectively assessing the treatment potential of new therapies across multiple subgroups.
In summary, umbrella trials like FUTURE, and NeoTRIP are at the forefront of precision medicine, allowing for more nuanced and effective approaches to breast cancer treatment. By evaluating multiple treatment options within specific molecular subtypes of a single disease, these trials not only accelerate the development of new therapies but also help to deliver the right treatment to the right patient at the right time.
Platform design
Platform design aims to study multiple targeted therapies in the context of a single disease in a perpetual manner, with therapies allowed to enter or leave the platform on the basis of a decision algorithm (Figure 3) (8).
(I) Platform design for breast cancer
When it comes to long-standing clinical trials in breast cancer, I-SPY 2 (NCT01042379) (25) holds an important place. This open-label, multi-center phase II platform trial has been ongoing for a decade, making it one of the longest-running trials designed with a platform concept. The use of a platform design in I-SPY 2 has provided a unique opportunity for studying various treatments within the same trial over an extended period, lending invaluable insights into breast cancer treatment.
The I-SPY 2 trial utilizes a Bayesian algorithm to enable dynamic randomization of treatment groups. This method allows for a continual learning process, wherein data from patients already enrolled in the trial contribute to decisions about the treatment assignment for future patients. With this innovative design, I-SPY 2 has heralded the successful application of platform design principles in the development of therapies for breast cancer.
The implementation of platform trials like I-SPY 2 underlines the advancement in clinical trial design, highlighting its potential to expedite the evaluation of new treatments and combination regimens. With dynamic randomization and adaptive features, these trials are likely to be instrumental in driving progress in the field of breast cancer therapeutics.
The accuracy in the precision of design: the shift towards more targeted approaches
Having discussed the broad advancements introduced by master protocols in the realm of clinical trial design, it is important to delve further into the precision these innovative methodologies can offer. In today’s era of precision tumor (10), it is feasible to determine the molecular phenotype of patients on an individual basis. More and more clinical trials are driven by biomarkers, and the fundamental objective of research is to identify the best recipients of experimental drugs and individualized therapy, Accurate screening of potential beneficiaries through effective biomarkers is helpful to improve the success rate of clinical trials, and at the same time, it can avoid exposing patients with little possibility of benefit to unnecessary safety risks. Under the premise of reasonable design and sufficient resources, clinical research driven by biomarkers can effectively and efficiently provide evidence for individualized treatment of patients. Compared with the traditional study of curative effect evaluation based on specific histopathological classification, the design and analysis plan of clinical research driven by biomarkers need to consider the nature of markers, detection accuracy and clinical practicability.
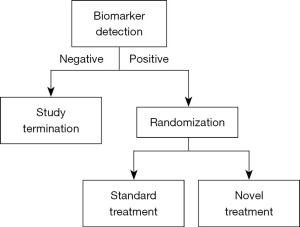
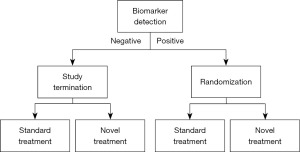
Enrichment design and marker stratified design are two popular biomarker-driven clinical research methods that can be used to precisely identify the recipients of malignancy treatment (Figures 4,5). The former is randomized only for the population with positive markers, while the latter is randomized separately based on the stratification of markers, which is actually randomized for the whole population. When there are multiple biomarkers, an experiment can also combine different basic designs, such as enrichment for one marker and randomization and stratification for all people for another marker. For example, many studies related to programmed death protein 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors. Enriched the EGFR-negative group first, then stratified and randomized the expression level of PD-L1.
Enrichment design
Enrichment design (26) in clinical trials is a potent strategy focusing on identifying and enrolling participants who are most likely to benefit from a certain experimental treatment based on specific characteristics such as genomics, pathophysiology, or histology. This design emphasizes on harnessing the power of biomarkers to predict and enhance the therapeutic effect of interventions in clinical trials, thereby potentially elevating the success rate.
Despite its clear advantages, enrichment design also presents challenges. Its effectiveness is largely reliant on the precision of biomarker detection-both in terms of sensitivity and specificity. Moreover, the translatability of the experimental results into clinical practice, as well as the applicability and extrapolation of findings, should be carefully considered.
(I) Enrichment design for breast cancer
Examining breast cancer trials such as the EMBRACA (27,28) and OlympiA (29) provides valuable insight into the implementation and impact of enrichment design. These trials, while demonstrating promising potential for improved patient outcomes, also highlight potential hurdles, including the lack of a significant improvement in overall survival and the impact on patient quality of life. Thus, while the enrichment design marks a significant stride in personalized medicine, it also calls for continuous improvement and refinement in its application.
Marker stratified designs
Marker-stratified design in clinical trials (30) is an effective approach that divides patients into different groups based on their biological markers. This includes diagnostic, prognostic, predictive, pharmacodynamic, safety, and monitoring biomarkers. Prognostic biomarkers in particular are often employed as stratification factors in clinical trials to discern populations with the same diagnosis but diverse natural disease progressions without treatment intervention. The use of such biomarkers can minimize subject variability, reduce the effect of confounding variables on the results, and mitigate bias between groups.
There are inherent challenges in marker-stratified designs. The primary endpoint can either be a positive subgroup or the overall population, which can lead to complexities in study design. If a positive subgroup is set as one of the primary or key secondary study endpoints, it’s vital to ensure sufficient sample size, lending confidence that the marker-positive subgroup will significantly benefit from the new therapy. To safeguard against inflation of class I error when designing multiple primary study endpoints, parallel strategies that split and recycle class I error or a fixed-order sequential test strategy that keeps α constant can be employed.
(I) Marker stratified designs for breast cancer
An illustrative example of marker-stratified design is the SOLAR-1 (NCT02437318) (31) phase 3 trial. This trial compared the efficacy and safety of alpelisib plus fulvestrant against placebo plus fulvestrant in patients with HR-positive, HER2-negative advanced breast cancer who had previously undergone endocrine therapy. Patients were stratified based on their tumor-tissue PIK3CA mutation status, with the primary endpoint being progression-free survival. Notably, in patients with PIK3CA-mutated cancer, the alpelisib-fulvestrant group demonstrated significantly longer progression-free survival.
However, while the treatment led to prolonged progression-free survival in patients with PIK3CA-mutated, HR-positive, HER2-negative advanced breast cancer, it was also accompanied by notable adverse events. Hyperglycemia and rash were the most common adverse events of grade 3 or 4, leading to a significant percentage of patients discontinuing treatment. Therefore, while marker-stratified designs hold substantial promise in tailoring treatments, careful consideration of potential adverse effects is crucial in realizing their full potential.
Discussion
Recap of the progress in clinical trial design for breast cancer
The evolution of clinical trial design in breast cancer has been remarkable. From conventional phase-based trials to innovative seamless, master protocol, and precision designs, each approach aims to accelerate and optimize research.
While traditional phase I–IV trials laid the groundwork, their limitations fuelled the emergence of more efficient designs (6). Seamless trials with their condensed phases address the time-lag in conventional models. However, they call for meticulous planning to ensure validity and safety (12).
Master protocols unlock invaluable synergies through their “sharing” philosophy (18). Basket trials extend drug evaluation across cancer subtypes, umbrella trials assess therapies within a subtype, and platform trials enable perpetual learning. Yet complexity in implementation remains a hurdle (16,32).
Precision designs leverage biomarkers to refine the trial strategy (33,34). Enrichment designs focus on likely responder subgroups, demanding robust assay sensitivity and specificity. Marker-stratified designs facilitate comparisons across biomarker-defined strata but require astute statistical considerations in trial design.
Each design confers unique strengths while confronting inherent challenges (32). A balanced appraisal shows traditional phase-based trials have merits in their structure and acceptance. Seamless and master protocols boost efficiency but require expertise in specialized statistical and bioinformatics approaches. Precision designs bring us closer to individualized treatment but hinge on biomarker validation (33).
Ultimately, fidelity to sound science and ethics is imperative regardless of design choice. Ongoing refinements in statistical rigor, bioinformatics capabilities and ethical safeguards will shape further progress. An integrated approach combining complementary designs may offer the best solution. Through it all, patient benefit should remain the guiding compass.
Current challenges and future perspectives
The path to optimizing clinical trial design for breast cancer remains strewn with hurdles. Key challenges span ethical, logistical, statistical, and bioinformatics domains.
Ethical concerns around risk-benefit ratios and informed consent are magnified in seamless and master protocols. Guarding patient safety and autonomy will be vital as complex designs gain traction (35).
Logistical difficulties in coordinating intricate trial workflows and recruiting adequate patient populations, especially for precision designs, need resolving. Platform designs call for long-term commitment (34).
Statistical complexities arise in data analysis and interpretation when dealing with vast heterogeneity, multiple arms and adaptive features. Close collaboration with biostatisticians is essential.
Robust bioinformatics infrastructure is indispensable, particularly with deep molecular profiling. Integrating and analyzing multilayered data meaningfully is the goal.
Future progress necessitates convergent efforts to tackle these challenges through guidelines, education, technological advances and global collaboration. Some promising directions include:
- Refining ethical frameworks attuned to innovative designs.
- Investment in training in specialized competencies like biostatistics, bioinformatics and computational methodology.
- Leveraging artificial intelligence and machine learning to enable platform trials and dynamic arms.
- Building centralized data sharing platforms to power basket, umbrella and platform designs.
- Exploring hybrid designs that optimize synergy.
While challenges remain, the outlook is bright. Continued cross-disciplinary collaboration, technological progress and evolving regulatory climate should usher in the next generation of clinical trials, taking us closer to precision oncology. With patient benefit the ultimate objective, resolute efforts to implement innovative designs will pave the way forward.
Conclusions
In conclusion, clinical trial design in breast cancer has undergone pivotal transformation, from conventional phase-based trials to innovative seamless, master protocol, and precision designs. This signifies a major shift towards more efficient and targeted research strategies. While challenges around ethics, logistics, and data complexity remain, these cutting-edge designs represent a tremendous leap towards personalized medicine. We look forward to witnessing their widespread implementation in breast cancer research through continual optimization, ultimately translating to patient benefit.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-22/rc
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-22/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-22/coif). JZ serves as an unpaid editorial board member of Translational Breast Cancer Research from May 2023 to April 2025. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Breast cancer [Internet]. [cited 2023 Aug 3]. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- Nolan E, Lindeman GJ, Visvader JE. Deciphering breast cancer: from biology to the clinic. Cell 2023;186:1708-28. [Crossref] [PubMed]
- Unger JM, Cook E, Tai E, et al. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ Book 2016;35:185-98. [Crossref] [PubMed]
- Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature 2012;483:531-3. [Crossref] [PubMed]
- Gan HK, You B, Pond GR, et al. Assumptions of expected benefits in randomized phase III trials evaluating systemic treatments for cancer. J Natl Cancer Inst 2012;104:590-8. [Crossref] [PubMed]
- Woodcock J, LaVange LM. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N Engl J Med 2017;377:62-70. [Crossref] [PubMed]
- National Medical Products Administration [Internet]. [cited 2023 Aug 3]. Available online: http://english.nmpa.gov.cn/
- Fountzilas E, Tsimberidou AM, Vo HH, et al. Clinical trial design in the era of precision medicine. Genome Med 2022;14:101. [Crossref] [PubMed]
- Roberts C, Torgerson DJ. Baseline imbalance in randomised controlled trials. BMJ 1999;319:185. [Crossref] [PubMed]
- Seamless Clinical Trials: Why Didn’t We Think Of That? | Premier Consulting [Internet]. [cited 2023 Aug 3]. Available online: https://premierconsulting.com/resources/blog/seamless-clinical-trials/
- Food and Drug Administration. Expansion Cohorts: Use in First-in-Human Clinical Trials to Expedite Development of Oncology Drugs and Biologics; 2022.
- Hobbs BP, Barata PC, Kanjanapan Y, et al. Seamless Designs: Current Practice and Considerations for Early-Phase Drug Development in Oncology. J Natl Cancer Inst 2019;111:118-28. [Crossref] [PubMed]
- National Library of Medicine, National Institute of Health. ClinicalTrials.gov website. Available online: https://clinicaltrials.gov. Accessed August 24, 2018.
- Bogin V. Master protocols: New directions in drug discovery. Contemp Clin Trials Commun 2020;18:100568. [Crossref] [PubMed]
- Renfro LA, Sargent DJ. Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples. Ann Oncol 2017;28:34-43. [Crossref] [PubMed]
- Redman MW, Allegra CJ. The Master Protocol Concept. Semin Oncol 2015;42:724-30. [Crossref] [PubMed]
- Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov 2023;22:101-26. [Crossref] [PubMed]
- Nardin S, Del Mastro L. Sacituzumab Govitecan in HR-positive HER2-negative metastatic breast cancer. Ann Transl Med 2023;11:228. [Crossref] [PubMed]
- Pfizer. A Phase 1b/2 study to evaluate safety and anti tumor activity of avelumab in combination with the Poly(Adenosine Diphosphate [ADP]-Ribose) Polymerase (PARP) inhibitor talazoparib in patients with locally advanced or metastatic solid tumors [Internet]. clinicaltrials.gov; 2023 May [cited 2023 Aug 2]. Report No.: NCT03330405. Available online: https://clinicaltrials.gov/study/NCT03330405
- American Society of Clinical Oncology. Targeted Agent and Profiling Utilization Registry (TAPUR) Study [Internet]. clinicaltrials.gov; 2023 Jul [cited 2023 Aug 2]. Report No.: NCT02693535. Available online: https://clinicaltrials.gov/study/NCT02693535
- Liu Y, Zhu XZ, Xiao Y, et al. Subtyping-based platform guides precision medicine for heavily pretreated metastatic triple-negative breast cancer: The FUTURE phase II umbrella clinical trial. Cell Res 2023;33:389-402. [Crossref] [PubMed]
- Yap TA, Bardia A, Dvorkin M, et al. Avelumab Plus Talazoparib in Patients With Advanced Solid Tumors: The JAVELIN PARP Medley Nonrandomized Controlled Trial. JAMA Oncol 2023;9:40-50. [Crossref] [PubMed]
- Wang H, Yee D. I-SPY 2: a Neoadjuvant Adaptive Clinical Trial Designed to Improve Outcomes in High-Risk Breast Cancer. Curr Breast Cancer Rep 2019;11:303-10. [Crossref] [PubMed]
- Simon N, Simon R. Adaptive enrichment designs for clinical trials. Biostatistics 2013;14:613-25. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- Litton JK, Hurvitz SA, Mina LA, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol 2020;31:1526-35. [Crossref] [PubMed]
- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021;384:2394-405. [Crossref] [PubMed]
- Halabi S, Lin CY, Liu A. On the design and the analysis of stratified biomarker trials in the presence of measurement error. Stat Med 2021;40:2783-99. [Crossref] [PubMed]
- André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2019;380:1929-40. [Crossref] [PubMed]
- Lu CC, Li XN, Broglio K, et al. Practical Considerations and Recommendations for Master Protocol Framework: Basket, Umbrella and Platform Trials. Ther Innov Regul Sci 2021;55:1145-54. [Crossref] [PubMed]
- Heckman-Stoddard BM, Smith JJ. Precision medicine clinical trials: defining new treatment strategies. Semin Oncol Nurs 2014;30:109-16. [Crossref] [PubMed]
- Hu C, Dignam JJ. Biomarker-Driven Oncology Clinical Trials: Key Design Elements, Types, Features, and Practical Considerations. JCO Precis Oncol 2019;3:PO.19.00086.
- Complex clinical trials – Questions and answers. Version 2022-05-23. [Internet]. [cited 2023 Aug 3]. Available online: https://health.ec.europa.eu/system/files/2022-06/medicinal_qa_complex_clinical-trials_en.pdf
Cite this article as: Zhou T, Zhang J. Types and progress of clinical trial design for breast cancer: a narrative review. Transl Breast Cancer Res 2023;4:20.