
Reporting breast density on chest computed tomography
Introduction
High breast density on mammography increases a woman’s risk of breast cancer by 2–6 times compared to less dense breast tissue (1). Dense breast tissue also reduces the sensitivity of two-dimensional mammography (2). Women with dense breast parenchyma may benefit from additional screening tests including ultrasound (3). Cancers occurring in areas of high breast density are associated with larger tumors, more positive lymph nodes, and negative estrogen receptors (4). Genetic and environmental factors associated with breast density include race, ethnicity, parity, diet, drugs, and hormones (5). In recent years, breast density is considered a partially modifiable risk factor (6). Breast density can be used as a guide for personalized screening strategies; it can guide lifestyle changes and possible drug interventions (7,8). Due to the impact of breast density on breast cancer risk, federal legislation recommends notifying both providers and patients about breast density on mammography (9).
Physics of mammography
Density differences provide the basis for radiographic and computed tomography (CT) imaging. The fundamental mechanism of image production for these modalities is the differential attenuation of the radiation beam as it passes through a subject (10). The attenuation coefficient of a material characterizes how easily a beam penetrates a given volume of a substance. In the context of medical imaging, attenuation differences in materials ultimately manifest as different shades of grey on images (11). At the molecular level, the density of the material through which the incident beam travels dictates the degree of beam attenuation; the dense electron cloud of more dense material will result in an increased attenuation of incident particles. The interaction of the incident beam and electrons results in the absorption or scattering of the incident beam, ultimately preventing it from striking the receptor. Together, the areas with more and fewer incident beams striking the receptor create the image. Materials with a greater atomic number have a greater electron field to balance the element’s charge and provide opportunities for more interactions with the incident beam resulting in more beam attenuation in elements with higher atomic numbers with fewer incident X-rays striking the detector (12). Five major densities are recognized on X-ray, from least to most dense: air, fat, water/soft tissue, bone, and metal (13).
Both normal breast tissue and breast cancer are soft tissue densities and therefore are more difficult to differentiate than structures of different densities, such as bone and soft tissue. In order to demonstrate differences in these tissues of similar attenuation, special conditions must be used during mammography to accentuate the small differences in tissue attenuation between normal breast tissue and breast cancer. The principal difference between mammography and digital radiography is the use of molybdenum and rhodium targets as opposed to tungsten targets for X-ray generation (14). Both molybdenum and rhodium are lower kilovoltage peak (kVp) materials, allowing differentiation of small differences in attenuation.
Evolution of breast density reporting on mammography
As mammography utilization increased in the 1980s, a wide variability of practices existed and were cited as substantial problems (15). In response, the American College of Radiology (ACR) convened a committee of radiologists, medical physicists, and a US Food and Drug Administration (FDA) representative to develop a voluntary mammography accreditation program in 1986 (16). A separate ACR committee also was charged with drafting guidelines on mammography reporting and management under the title of the Breast Imaging Reporting and Data System (BI-RADS) (17). The original BI-RADS document described the overall structure of the breast imaging report, which included a summary of breast density, a description of significant findings (using appropriate descriptors as well as size and location), and a final assessment and management section.
The inclusion of a statement describing the general breast tissue type arose from evidence in the literature establishing that increased breast density is accompanied by decreased sensitivity (18,19). Over time, research has shown that increased breast density also is associated with increased breast cancer risk which remains an area of active research (20-22). The inclusion of 4 categories describing breast density (ranging from the almost entirely fatty breast to the extremely dense breast) in the standard mammography report is designed to improve the communication of predicted mammographic performance and breast cancer risk (23).
Introduced in the Senate on October 25, 2017, the Breast Density and Mammography Reporting Act of 2017 amended the Public Health Service Act to require mammography facilities to include up-to-date information about breast density in both the written report of the results of a mammography examination provided to a patient’s physician and the summary of that written report given to the patient. The summary must convey: (I) the effect of breast density in masking the presence of breast cancer on a mammogram; and (II) that individuals with dense breasts should talk with their healthcare providers about questions or concerns regarding the benefit of additional testing. The bill requires the Department of Health and Human Services to expand and intensify research on breast density, the cost-effectiveness and feasibility of supplemental imaging relating to breast density, and best practices concerning mammograms and supplemental screening for those with dense breasts (24).
Evolution of chest CT
In 1972, the first patient underwent a head CT scan and Godfrey Hounsfield received the Nobel Prize in physics for inventing computer tomography. He explained the limitations of two-dimensional imaging with deep objects overlapping superficial objects challenging the radiologist and that X-ray can only distinguish between white and black and cannot differentiate soft tissues. Hounsfield went further to say that CT allows one to study the nature of tissue (25). The Hounsfield unit (HU) is the CT measurement of density obtained from the linear transformation of attenuation coefficients (26). Similar to measurements of density based on kVp in radiography, HUs are not constant but vary as a result of changes in beam energy. By convention, 0 HU is defined as water, −1,000 is defined as air, and 1,000 bone (27). Therefore, in comparison to the five major densities on X-ray, CT can assign densities ranging from −1,000 to +1,000 allowing far better tissue discrimination. Breast density can be readily determined on chest CT currently. In the future, given the increased granularity of chest CT, improved discrimination of breast cancer risk may be possible.
Breast density on chest CT
The breasts are exposed to radiation during chest CT because they are within the CT gantry so therefore, they should be included on images provided to the radiologist (28). At our institution, we request that patients keep their bras in place during CT so that the breast tissue is reproducibly located within the field of view which has fortuitously been shown to decrease radiation dose to the breast parenchyma (29). Margolies and colleagues emphasized on breast cancer detection on CT (30). Our previous work demonstrated that breast density readings on chest CT agreed with mammographic breast density readings and in fact, there was a greater inter-reader agreement for breast density on chest CT than on mammography. Computer analysis of the same patients yielded comparable results to CT visual readings (31). Furthermore, general radiologists were also able to achieve substantial to excellent agreement (32). Figure 1 demonstrates that breast density on chest CT is analogous to breast density on mammography.
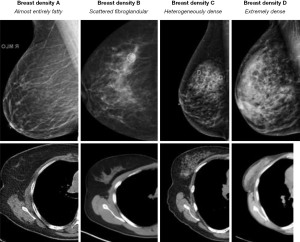
Conclusions
The number of chest CT scans performed each year is increasing. Chest CT scans for lung cancer screening in high-risk patients are the standard of care. Important additional findings can be identified on these exams including coronary artery calcifications, thyroid nodules, and breast cancer (33). Furthermore, high breast density can be diagnosed, which puts a woman at greater risk of developing breast cancer. It is important that thoracic radiologists include the grading of breast density in their reports because this information has been proven to be helpful for early detection of breast cancer.
There are challenges that need to be considered. First, the chest CT needs to include the entire breast in order to make an accurate assessment of breast density. If the entire breast is not included the density should not be reported. However, the breast is exposed to radiation during the scan and should be evaluated; evaluation of the breast tissue is optimized with a bra in place that diminishes the radiation dose because of positioning and is more comfortable for the patient. Second, it will require additional education for non-mammographers to measure breast density on chest CT. However, in our study with seven general radiologists, the agreement with experts was substantial to excellent with kappa statistics of 0.61–0.88. Third, the clinical implications of knowing about a women’s risk for breast cancer are empowering for the patient. Breast density is considered a partially modifiable risk factor and knowledge of breast density can guide lifestyle changes and possible drug interventions. Federal legislation recommends notifying both providers and patients about breast density on mammography and so it follows that if we see the same information on chest CT, we should report it so that at the very least the clinician can encourage their patient to have a routine mammogram.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-36/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-36/coif). MMS reports grant funding from Boehringer Ingelheim and Genentech, research with Bioclinica, LungLifeAI, AbbVie, and speaking with Peer View and France Foundation. KMC served as an advisor for Cardinal Health Oncology Summits. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Landsmann A, Ruppert C, Wieler J, et al. Radiomics in photon-counting dedicated breast CT: potential of texture analysis for breast density classification. Eur Radiol Exp 2022;6:30. [Crossref] [PubMed]
- Nickel B, Copp T, Li T, et al. A systematic assessment of online international breast density information. Breast 2022;65:23-31. [Crossref] [PubMed]
- Haas JS, Giess CS, Harris KA, et al. Randomized Trial of Personalized Breast Density and Breast Cancer Risk Notification. J Gen Intern Med 2019;34:591-7. [Crossref] [PubMed]
- Gutiérrez-Lopez M, Prado-Olivarez J, Matheus-Troconis C, et al. A Case Study in Breast Density Evaluation Using Bioimpedance Measurements. Sensors (Basel) 2022;22:2747. [Crossref] [PubMed]
- Shamsi U, Afzal S, Shamsi A, et al. Factors associated with mammographic breast density among women in Karachi Pakistan. BMC Womens Health 2021;21:438. [Crossref] [PubMed]
- Darcey E, McCarthy N, Moses EK, et al. Is Mammographic Breast Density an Endophenotype for Breast Cancer? Cancers (Basel) 2021;13:3916. [Crossref] [PubMed]
- Fowler EE, Smallwood A, Khan N, et al. Calibrated Breast Density Measurements. Acad Radiol 2019;26:1181-90. [Crossref] [PubMed]
- Hwang KT, Chu AJ, Kim J, et al. Prognostic Influence of Preoperative Mammographic Breast Density in Operable Invasive Female Breast Cancer. Sci Rep 2018;8:16075. [Crossref] [PubMed]
- Rhodes DJ, Jenkins SM, Hruska CB, et al. Breast Density Awareness, Knowledge, and Attitudes Among US Women: National Survey Results Across 5 Years. J Am Coll Radiol 2020;17:391-404. [Crossref] [PubMed]
- Seibert JA. X-ray imaging physics for nuclear medicine technologists. Part 1: Basic principles of x-ray production. J Nucl Med Technol 2004;32:139-47.
- Pooley RA, McKinney JM, Miller DA. The AAPM/RSNA physics tutorial for residents: digital fluoroscopy. Radiographics 2001;21:521-34. [Crossref] [PubMed]
- Webb S. The Physics of Medical Imaging. 1st ed. Boca Raton: CRC Press, 1988.
- Myer W. Radiography review: radiographic density. Veterinary Radiology 1977;18:138-40.
- Wu X, Gingold EL, Barnes GT, et al. Normalized average glandular dose in molybdenum target-rhodium filter and rhodium target-rhodium filter mammography. Radiology 1994;193:83-9. [Crossref] [PubMed]
- Conway BJ, McCrohan JL, Rueter FG, et al. Mammography in the eighties. Radiology 1990;177:335-9. [Crossref] [PubMed]
- McLelland R, Hendrick RE, Zinninger MD, et al. The American College of Radiology Mammography Accreditation Program. AJR Am J Roentgenol 1991;157:473-9. [Crossref] [PubMed]
- D'Orsi CJ, Kopans DB. Mammography interpretation: the BI-RADS method. Am Fam Physician 1997;55:1548-50, 1552.
- Fajardo LL, Hillman BJ, Frey C. Correlation between breast parenchymal patterns and mammographers' certainty of diagnosis. Invest Radiol 1988;23:505-8. [Crossref] [PubMed]
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000;92:1081-7. [Crossref] [PubMed]
- Hainline S, Myers L, McLelland R, et al. Mammographic patterns and risk of breast cancer. AJR Am J Roentgenol 1978;130:1157-8. [Crossref] [PubMed]
- Whitehead J, Carlile T, Kopecky KJ, et al. Wolfe mammographic parenchymal patterns. A study of the masking hypothesis of Egan and Mosteller. Cancer 1985;56:1280-6. [Crossref] [PubMed]
- Carlile T, Kopecky KJ, Thompson DJ, et al. Breast cancer prediction and the Wolfe classification of mammograms. JAMA 1985;254:1050-3.
- Burnside ES, Sickles EA, Bassett LW, et al. The ACR BI-RADS experience: learning from history. J Am Coll Radiol 2009;6:851-60. [Crossref] [PubMed]
- H.R.4122-115th Congress (2017-2018): Breast Density and Mammography Reporting Act of 2017. Available online: https://www.congress.gov/bill/115th-congress/house-bill/4122/text
- Hounsfield GN. Computed medical imaging. Nobel lecture, Decemberr 8, 1979. J Comput Assist Tomogr 1980;4:665-74. [Crossref] [PubMed]
- Razi T, Niknami M, Alavi Ghazani F. Relationship between Hounsfield Unit in CT Scan and Gray Scale in CBCT. J Dent Res Dent Clin Dent Prospects 2014;8:107-10. [Crossref] [PubMed]
- Lamba R, McGahan JP, Corwin MT, et al. CT Hounsfield numbers of soft tissues on unenhanced abdominal CT scans: variability between two different manufacturers' MDCT scanners. AJR Am J Roentgenol 2014;203:1013-20. [Crossref] [PubMed]
- Salvatore M, Margolies L, Bertolini A, et al. The need to be all inclusive: Chest CT scans should include imaged breast parenchyma. Clin Imaging 2018;50:243-5. [Crossref] [PubMed]
- Seidenfuss A, Mayr A, Schmid M, et al. Dose reduction of the female breast in chest CT. AJR Am J Roentgenol 2014;202:W447-52. [Crossref] [PubMed]
- Margolies LR, Salvatore M, Yip R, et al. The chest radiologist's role in invasive breast cancer detection. Clin Imaging 2018;50:13-9. [Crossref] [PubMed]
- Salvatore M, Margolies L, Kale M, et al. Breast density: comparison of chest CT with mammography. Radiology 2014;270:67-73. [Crossref] [PubMed]
- Margolies L, Salvatore M, Eber C, et al. The general radiologist's role in breast cancer risk assessment: breast density measurement on chest CT. Clin Imaging 2015;39:979-82. [Crossref] [PubMed]
- Yip R, Jirapatnakul A, Hu M, et al. Added benefits of early detection of other diseases on low-dose CT screening. Transl Lung Cancer Res 2021;10:1141-53. [Crossref] [PubMed]
Cite this article as: Beizavi Z, Desperito E, Capaccione KM, Patrizio R, Salvatore MM. Reporting breast density on chest computed tomography. Transl Breast Cancer Res 2023;4:24.


