
A clinical perspective on oncoplastic breast conserving surgery
Introduction
Breast conserving surgery (BCS) plus radiation treatment is the favored alternative for mastectomy in patients with breast cancer (1-9). Nowadays, around two-thirds of breast cancer patients undergo BCS (10). Since the survival of breast cancer patients increases, quality of life (QoL) after breast cancer (treatment) becomes more important. With the shift towards more BCS, there is a growing emphasis on aesthetic outcomes after surgery, which is an important aspect of QoL (11).
The challenges for standard BCS (s-BCS) are seen in patients with large invasive tumors that did not respond well on neoadjuvant systemic treatment (NST) and in patients with extensive ductal carcinoma in situ (DCIS). In both situations, there is an unfavorable tumor-to-breast volume ratio. In these cases, maintaining both oncological safety and aesthetic outcomes with s-BCS is difficult. To allow for breast conservation in such patients, oncoplastic breast conserving surgery (OPBCS) techniques are introduced. The goal of OPBCS is to combine the oncological tumor excision with plastic surgical breast conservation techniques, without compromising oncological safety and maintaining aesthetic outcomes by preserving the shape of the breast (12-17).
The first techniques used for OPBCS were introduced at the end of the previous century (18-20). Since then, a broad range of techniques have been developed. Despite the promising role OPBCS plays in clinical practice, the demand for standardization is recognized (21-25). At the same time, the adoption rates of OPBCS are relatively slow (24). This article provides a clinical perspective on OPBCS.
Definitions
Recently, the American Society of Breast Surgeons (ASBrS) developed a consensus on the definition and classification system for OPBCS (21). OPBCS involves the combination of an oncological resection with volume displacement or volume replacement techniques to reconstruct the breast. The various definitions used in this article can be found in Table 1.
Table 1
| Classification | Definition |
|---|---|
| OPBCS | OPBCS involves the combination of an oncological resection with volume displacement or volume replacement techniques to reconstruct the breast |
| Level I | <20% of breast volume excised, no skin excision required, no mammoplasty techniques required |
| - Simple volume displacement | |
| Level II | 20–50% of breast volume excised |
| Excision of excess skin required, based on mammoplasty techniques | |
| - Volume displacement technique (reduction) | |
| - Volume replacement, autologous tissue from outside the breast is used for reconstruction and volume compensation |
OPBCS, oncoplastic breast conserving surgery.
OPBCS is mostly considered if there is a significant dead space remaining after the excision of the tumor (26,27). If the excised volume is lower than 20% of the total volume of the breast, no large skin excision is required, and no further mammoplasty techniques are necessary, other than simple volume displacement techniques (level-1 OPBCS). When 20% to 50% of the breast volume is excised (level-2 OPBCS), excision of excess skin is required and reduction with mammoplasty techniques may be applied (28).
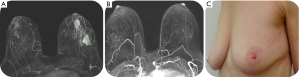
In case of volume displacement in level-2 OPBCS, breast tissue from the same breast is used, and replaced into the surgical defect (Figure 1, patient A). With volume replacement techniques, autologous tissue from outside the breast is used for reconstruction and volume compensation, such as the latissimus dorsi (LD) flap, local perforator flaps [e.g., lateral and anterior intercostal artery perforator flaps (LICAP and AICAP), thoracodorsal artery perforator flap (TDAP, also shown in Figure 2, patient B), internal mammary artery perforator flap (IMAP) or the thoraco-abdominal flap (TAP)].
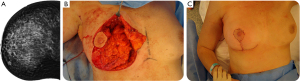
The indication for, and selection of the appropriate reconstruction technique depends on personal preferences and expertise of the MDT (20,26,29-31).
Current use of OPBCS
Despite the proven benefits of OPBCS regarding cosmetic outcomes and patient satisfaction, the increase in the adoption of OPBCS is relatively low (24). Kimball et al. showed that, although the high number of s-BCS performed annually in the US, the annual growth rate of OPBCS is less than 10% in the last 6 years (24). Large variation in its use, varying from nineteen percent in the Pacific to only three percent in the East South Central of the US, illustrates the need for standardization and awareness among breast cancer surgeons. Standardization of the goals and indications for OPBCS is important to optimize patient selection and for adequate evaluation of cosmetic- and oncologic outcomes and QoL (22,32). Most surgeons agree that the objectives of OPBCS include broadening the indications for s-BCS to include patients with large and/or multifocal tumors as an alternative to mastectomy, enhancing cosmetic outcomes, improving QoL, and reducing reoperations due to positive margins (22). Indication for OPBCS is preferably discussed within a MDT including a plastic surgeon and radiation oncologist. OPBCS is mostly considered in patients with significant dead space remaining after s-BCS or when more than 20% of the breast volume needs to be excised (27).
Volume displacement techniques (e.g., breast reduction techniques) can be used in patients in whom s-BCS would result in nipple malposition, breast hypertrophy, breast ptosis and in patients with a tumor located primarily in the upper quadrant of the lower half of the breast (27). In these patients, reduction of the contralateral breast is often necessary to obtain symmetry.
Volume replacement techniques are indicated in patients to ensure the preservation of shape and sufficient volume of the breast that cannot be achieved with volume displacement techniques and for those who do not want smaller breasts (27).
OPBCS can be performed in different stages during breast cancer treatment: in the primary surgery setting (Figure 1) as well as after NST (Figure 2). OPBCS can be performed as one-step procedure (= immediate OPBCS) or two-step procedure (= delayed OPBCS).
Immediate OPBCS has the advantage that the oncological excision and reconstruction are performed in one procedure. However, in case of positive resection margins requiring re-excision, a cosmetically successful reconstruction may have to be dismantled when re-excision is required. Moreover, re-excisions due to positive resection margins increase the risk of complications such as surgical site infections (SSIs), impaired cosmetic outcome and may delay adjuvant treatment (33-36). Thus, patients with a high risk for positive resection margins after s-BCS could benefit from delayed instead of immediate OPBCS. Risk factors for positive resection margins include multifocal breast cancer, invasive lobular carcinoma (ILC), larger tumors and DCIS (Figures 1,2) (37). Van Loevezijn et al. furthermore showed that risk factors differ between patients treated with and without neoadjuvant chemotherapy, except for ILC (38).
Delayed OPBCS (Figure 3) can be explained as a two-step OPBCS: the first surgery aims for a radical excision of the tumor. The second surgery is already planned within a short period of time (e.g., within 2 to 3 weeks) enabling re-excision if necessary based on the histology report, prior to the oncoplastic procedure.
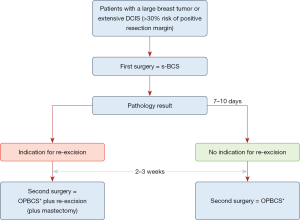
Van Loevezijn et al. recently investigated the timing of OPBCS and its short-term surgical outcomes (38). If the risk of positive resection margins after s-BCS was deemed (>30%) significant, patients were selected for delayed OPBCS. Results of this study showed that both immediate- and delayed OPBCS allowed breast conservation in 97% of all cases (n=251) with a low complication rate (3%). Despite a re-excision rate of 66% in the delayed-OPBCS group, the breast-conserving rate was high (93%).
One of the disadvantages of this two-step approach in delayed OPBCS is the need for a second operation. However, if there is an indication for an oncological re-excision, dismantling the potential reconstruction is avoided. Another potential downside of the two-step approach is that a second operation may be associated with an increased risk of postoperative wound healing (31,39-42).
Outcomes
Surgical outcomes
Regarding surgical outcomes of OPBCS, such as excision margins, re-excision rates and short-term surgical complications, the results seem to be in advantage compared to s-BCS. Heeg et al. reported on the excision margins after OPBCS compared to s-BCS and showed a small difference in re-excision rates in favor of the OPBCS group (15.6% vs. 14.1%, P=0.012) with a similar conversion rate to mastectomy in both groups (3.7% vs. 3.2%, P=0.105) (43). Nanda et al. (16) recently published a Cochrane Review on 78 non-randomized cohort studies and their results on OPBCS. The re-excision rate for OPBCS compared to s-BCS was lower [risk ratio (RR) 0.76; 95% confidence interval (CI): 0.69–0.85]. In line with these results, the OPBC-01/iTOP study reported on the resection margins by comparing BCS/level-1 OPBCS with level-2 OPBCS. Between the groups, the proportion with a margin below one millimeter differed significantly (17% in BCS/level-1 OPBCS vs. 6% in level-2 OPBCS, P<0.001) (44). The re-excision rates due to positive margins was 11% in the BCS/level-1 OPBCS group vs. 7% in the level-2 OBPCS group (P=0.025). The non-inferiority of OPBCS to s-BCS alone regarding surgical outcomes is supported by a recently published consensus (45). Few studies reported on short-term surgical complication rates after OPBCS. The Cochrane Review emphasizes that OPBCS may increase the number of complications, and the number of recalls for biopsies (16). Carter et al. analyzed the complications of OPBCS compared to s-BCS and mastectomy with- and without reconstruction. In their study population (n=10,607), patients treated with OPBCS had few seromas and hematomas (13.4% and 1.9% vs. 18.0% and 2.5%, P≤0.05). Wound-related complications and SSIs were seen more often with OPBCS than s-BCS (4.8% and 4.5% vs. 1.4% and 4.1%, P≤0.05). Compared to patients who underwent a mastectomy with reconstruction, there were fewer wound-related complications and SSI in the OPBCS group (respectively 4.8% and 4.2% vs. 11.6% and 13.0%, P≤0.05) (13).
Oncological outcomes
Although the surgical outcomes are in favor of OPBCS, oncological outcomes appear not to differ from s-BCS (16). Multiple studies have shown no significant difference in local recurrence (LR) rates and overall survival (OS) of OPBCS compared to s-BCS without oncoplastic reconstruction (15,44,46,47).
There are several studies reporting on QoL and cosmetic satisfaction after OPBCS compared to s-BCS and both similar or improved results are described (48,49).
Losken et al. performed a meta-analysis comparing OPBCS with s-BCS in which they found a significantly higher patient satisfaction with aesthetic outcome after surgery in the OPBCS group compared to s-BCS (89.5% vs. 82.9%, P≤0.001) (50). In an observational study, Santos et al. compared 57 patients who underwent OPBCS with 65 patients undergoing s-BCS. Although oncological and plastic surgeons, as well as semiautomatic software, rated the aesthetic outcome in favor of OPBCS, patients did not report a significant difference in the aesthetic results between OPBCS and s-BCS (51). Recently, a Brazilian study was published comparing the patient-reported outcomes based on the BREAST-Q after OPBCS compared to mastectomy with reconstruction. In the OPBCS group, the satisfaction rates with the breast(s) were higher as well as psychosocial and sexual well-being (52).
In attempt to fill the gap regarding QoL, the ANTHEM study group is currently performing a prospective study on the outcomes of OPBCS to support informed decision making in the future (53).
Radiotherapy after OPBCS
Adjuvant RT plays a central role in breast conserving treatment (54). After OPBCS, recognizing the target area for adjuvant RT can potentially be difficult since breast tissue may be replaced from one side to another, or tissue is displaced. In some patients, a radiation boost is indicated to maintain local control of disease (55). However, boost therapy increases the chance on developing moderate to severe fibrosis (56). In OPBCS, the target area for RT, and thus for the boost, may increase due to surgical manipulation of tissue. Therefore, in all patients, but specifically in patients with an indication for boost therapy, the benefits of OPBCS should be discussed multidisciplinaryly, keeping both oncological and aesthetic outcomes in mind. Metz et al. (57) reported on some crucial multidisciplinary recommendations when considering OPBCS:
- Consultation by a radiation oncologist pre-operative is advised.
- Discuss the eligibility for OPBCS in a MDT.
- The use of surgical clips enables precise target delineation during radiation therapy (planning) (58). Surgical clips should, at least, be placed on all sides of the cavity. Placing the marking clips ensures more accurate target volumes for post-operative radiotherapy, which reduces toxicity (26,58).
- The surgical report should consist of clear notations about the topographic location of resection planes of the lumpectomy cavity after reconstruction; the location of placed marking clips; the three-dimensional rearrangement of surrounding tissue; the marking of the specimen and in case of nipple displacement or re-centering, the vascularization of the nipple-areola complex needs to be reported (27).
- Oncological surgeons and radiation oncologists should speak each other’s language regarding the basic understanding of the surgical and radiation techniques.
Conclusions
In conclusion, OPBCS allows for breast conservation in a selective group of breast cancer patients who initially would have been treated with mastectomy due to the unfavorable tumor-to-breast ratio. Preserving the breast with OPBCS improves the QoL compared to mastectomy. In contrast to a recently published recommendation (45), our opinion is that OPBCS should not be applied to all breast cancer patients. Selection of patients who benefit from OPBCS as well as the timing of OPBCS techniques are best discussed in a MDT. Caution is required in patients with higher risk of positive margins (e.g., multifocal breast cancer, ILC, larger tumors and DCIS). In these patients, delayed OPBCS is recommended to facilitate re-excision and maintain excellent breast conserving rates.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-40/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-40/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg 2015;220:1063-9. [Crossref] [PubMed]
- van der Noordaa MEM, Ioan I, Rutgers EJ, et al. Breast-Conserving Therapy in Patients with cT3 Breast Cancer with Good Response to Neoadjuvant Systemic Therapy Results in Excellent Local Control: A Comprehensive Cancer Center Experience. Ann Surg Oncol 2021;28:7383-94. [Crossref] [PubMed]
- Puig CA, Hoskin TL, Day CN, et al. National Trends in the Use of Neoadjuvant Chemotherapy for Hormone Receptor-Negative Breast Cancer: A National Cancer Data Base Study. Ann Surg Oncol 2017;24:1242-50. [Crossref] [PubMed]
- Spronk PER, Volders JH, van den Tol P, et al. Breast conserving therapy after neoadjuvant chemotherapy; data from the Dutch Breast Cancer Audit. Eur J Surg Oncol 2019;45:110-7. [Crossref] [PubMed]
- van Bommel A, Spronk P, Mureau M, et al. Breast-Contour-Preserving Procedure as a Multidisciplinary Parameter of Esthetic Outcome in Breast Cancer Treatment in The Netherlands. Ann Surg Oncol 2019;26:1704-11. [Crossref] [PubMed]
- Gulis K, Ellbrant J, Svensjö T, et al. A prospective cohort study identifying radiologic and tumor related factors of importance for breast conserving surgery after neoadjuvant chemotherapy. Eur J Surg Oncol 2023;49:1189-95. [Crossref] [PubMed]
- Heeg E, Jensen MB, Mureau MAM, et al. Breast-contour preserving procedures for early-stage breast cancer: a population-based study of the trends, variation in practice and predictive characteristics in Denmark and the Netherlands. Breast Cancer Res Treat 2020;182:709-18. [Crossref] [PubMed]
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158-70. [Crossref] [PubMed]
- Fodor J, Major T, Tóth J, et al. Comparison of mastectomy with breast-conserving surgery in invasive lobular carcinoma: 15-Year results. Rep Pract Oncol Radiother 2011;16:227-31. [Crossref] [PubMed]
- van der Meer DJ, Kramer I, van Maaren MC, et al. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017. Int J Cancer 2021;148:2289-303. [Crossref] [PubMed]
- Markopoulos C, Tsaroucha AK, Kouskos E, et al. Impact of breast cancer surgery on the self-esteem and sexual life of female patients. J Int Med Res 2009;37:182-8. [Crossref] [PubMed]
- Shaitelman SF, Jeruss JS, Pusic AL. Oncoplastic Surgery in the Management of Breast Cancer. J Clin Oncol 2020;38:2246-53. [Crossref] [PubMed]
- Carter SA, Lyons GR, Kuerer HM, et al. Operative and Oncologic Outcomes in 9861 Patients with Operable Breast Cancer: Single-Institution Analysis of Breast Conservation with Oncoplastic Reconstruction. Ann Surg Oncol 2016;23:3190-8. [Crossref] [PubMed]
- Jonczyk MM, Jean J, Graham R, et al. Surgical trends in breast cancer: a rise in novel operative treatment options over a 12 year analysis. Breast Cancer Res Treat 2019;173:267-74. [Crossref] [PubMed]
- De La Cruz L, Blankenship SA, Chatterjee A, et al. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann Surg Oncol 2016;23:3247-58. [Crossref] [PubMed]
- Nanda A, Hu J, Hodgkinson S, et al. Oncoplastic breast-conserving surgery for women with primary breast cancer. Cochrane Database Syst Rev 2021;10:CD013658. [PubMed]
- Bolliger M, Lanmüller P, Schuetz M, et al. The iTOP trial: Comparing immediate techniques of oncoplastic surgery with conventional breast surgery in women with breast cancer - A prospective, controlled, single-center study. Int J Surg 2022;104:106694. [Crossref] [PubMed]
- Benelli L. A new periareolar mammaplasty: the "round block" technique. Aesthetic Plast Surg 1990;14:93-100. [Crossref] [PubMed]
- Galimberti V, Zurrida S, Zanini V, et al. Central small size breast cancer: how to overcome the problem of nipple and areola involvement. Eur J Cancer 1993;29A:1093-6. [Crossref] [PubMed]
- Clough KB, Lewis JS, Couturaud B, et al. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003;237:26-34. [Crossref] [PubMed]
- Chatterjee A, Gass J, Patel K, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3436-44. [Crossref] [PubMed]
- Weber WP, Soysal SD, El-Tamer M, et al. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Cancer Res Treat 2017;165:139-49. [Crossref] [PubMed]
- Maliko N, Schok T, Bijker N, et al. Oncoplastic Breast Conserving Surgery: Is There a Need for Standardization? Results of a Nationwide Survey. Breast Care (Basel) 2023;18:90-6. [Crossref] [PubMed]
- Kimball CC, Nichols CI, Vose JG, et al. Trends in Lumpectomy and Oncoplastic Breast-Conserving Surgery in the US, 2011-2016. Ann Surg Oncol 2018;25:3867-73. [Crossref] [PubMed]
- Heil J, Riedel F, Solbach C, et al. Oncoplastic breast-conserving surgery: More relevant than ever? Results of a survey among breast surgeons. Arch Gynecol Obstet 2019;299:1109-14. [Crossref] [PubMed]
- Richtlijnendatabase. Breast Reconstruction - Operation report for oncoplastic surgery. Accessed on 18-07-2023. Available online: https://richtlijnendatabase.nl/en/richtlijn/breast_reconstruction/incomplete_resection_oncoplastic_surgery.html
- Mureau MAM. Dutch breast reconstruction guideline. J Plast Reconstr Aesthet Surg 2018;71:290-304. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- Noguchi M, Saito Y, Mizukami Y, et al. Breast deformity, its correction, and assessment of breast conserving surgery. Breast Cancer Res Treat 1991;18:111-8. [Crossref] [PubMed]
- Kronowitz SJ, Hunt KK, Kuerer HM, et al. Practical guidelines for repair of partial mastectomy defects using the breast reduction technique in patients undergoing breast conservation therapy. Plast Reconstr Surg 2007;120:1755-68. [Crossref] [PubMed]
- Raufdeen F, Murphy J, Ahluwalia M, et al. Outcomes in volume replacement and volume displacement techniques in oncoplastic breast conserving surgery: A systematic review. J Plast Reconstr Aesthet Surg 2021;74:2846-55. [Crossref] [PubMed]
- Weber WP, Morrow M, Boniface J, et al. Knowledge gaps in oncoplastic breast surgery. Lancet Oncol 2020;21:e375-85. [Crossref] [PubMed]
- Fraser VJ, Nickel KB, Fox IK, et al. THE EPIDEMIOLOGY AND OUTCOMES OF BREAST CANCER SURGERY. Trans Am Clin Climatol Assoc 2016;127:46-58. [PubMed]
- Xue DQ, Qian C, Yang L, et al. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol 2012;38:375-81. [Crossref] [PubMed]
- O'Connell RL, Rattay T, Dave RV, et al. The impact of immediate breast reconstruction on the time to delivery of adjuvant therapy: the iBRA-2 study. Br J Cancer 2019;120:883-95. [Crossref] [PubMed]
- Olsen MA, Nickel KB, Margenthaler JA, et al. Increased Risk of Surgical Site Infection Among Breast-Conserving Surgery Re-excisions. Ann Surg Oncol 2015;22:2003-9. [Crossref] [PubMed]
- van Deurzen CH. Predictors of Surgical Margin Following Breast-Conserving Surgery: A Large Population-Based Cohort Study. Ann Surg Oncol 2016;23:627-33. [Crossref] [PubMed]
- van Loevezijn AA, Geluk CS, van den Berg MJ, et al. Immediate or delayed oncoplastic surgery after breast conserving surgery at the Netherlands Cancer Institute: a cohort study of 251 cases. Breast Cancer Res Treat 2023;198:295-307. [Crossref] [PubMed]
- Munhoz AM, Aldrighi CM, Montag E, et al. Outcome analysis of immediate and delayed conservative breast surgery reconstruction with mastopexy and reduction mammaplasty techniques. Ann Plast Surg 2011;67:220-5. [Crossref] [PubMed]
- Patel KM, Hannan CM, Gatti ME, et al. A head-to-head comparison of quality of life and aesthetic outcomes following immediate, staged-immediate, and delayed oncoplastic reduction mammaplasty. Plast Reconstr Surg 2011;127:2167-75. [Crossref] [PubMed]
- Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg 2006;117:1-11; discussion 12-4. [Crossref] [PubMed]
- Egro FM, Pinell-White X, Hart AM, et al. The use of reduction mammaplasty with breast conservation therapy: an analysis of timing and outcomes. Plast Reconstr Surg 2015;135:963e-71e. [Crossref] [PubMed]
- Heeg E, Jensen MB, Hölmich LR, et al. Rates of re-excision and conversion to mastectomy after breast-conserving surgery with or without oncoplastic surgery: a nationwide population-based study. Br J Surg 2020;107:1762-72. [Crossref] [PubMed]
- Fitzal F, Bolliger M, Dunkler D, et al. Retrospective, Multicenter Analysis Comparing Conventional with Oncoplastic Breast Conserving Surgery: Oncological and Surgical Outcomes in Women with High-Risk Breast Cancer from the OPBC-01/iTOP2 Study. Ann Surg Oncol 2022;29:1061-70. [Crossref] [PubMed]
- Rocco N, Catanuto G, Cinquini M, et al. Should oncoplastic breast conserving surgery be used for the treatment of early stage breast cancer? Using the GRADE approach for development of clinical recommendations. Breast 2021;57:25-35. [Crossref] [PubMed]
- André C, Holsti C, Svenner A, et al. Recurrence and survival after standard versus oncoplastic breast-conserving surgery for breast cancer. BJS Open 2021;5:zraa013.
- De Lorenzi F, Loschi P, Bagnardi V, et al. Oncoplastic Breast-Conserving Surgery for Tumors Larger than 2 Centimeters: Is it Oncologically Safe? A Matched-Cohort Analysis. Ann Surg Oncol 2016;23:1852-9. [Crossref] [PubMed]
- de Oliveira-Junior I, da Silva IA, da Silva FCB, et al. Oncoplastic Surgery in Breast-Conserving Treatment: Patient Profile and Impact on Quality of Life. Breast Care (Basel) 2021;16:243-53. [Crossref] [PubMed]
- Hernanz F, Jimeno J, Paz L, et al. Comparison of Conventional vs. Oncoplastic Breast Conserving Surgery in a Breast Unit with Oncoplastic Training. World J Surg Surgical Res 2021;4:1317.
- Losken A, Dugal CS, Styblo TM, et al. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014;72:145-9. [Crossref] [PubMed]
- Santos G, Urban C, Edelweiss MI, et al. Long-Term Comparison of Aesthetical Outcomes After Oncoplastic Surgery and Lumpectomy in Breast Cancer Patients. Ann Surg Oncol 2015;22:2500-8. [Crossref] [PubMed]
- Araújo Pereira Lisboa FC, Paulinelli RR, Campos Veras LP, et al. Aesthetic results were more satisfactory after oncoplastic surgery than after total breast reconstruction according to patients and surgeons. Breast 2023;71:47-53. [Crossref] [PubMed]
- Davies C, Whisker L, Skillman J, et al. Current practice and provision of oncoplastic breast-conserving surgery in the UK: results of the ANTHEM national practice questionnaire. Breast Cancer Res Treat 2023;200:163-70. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol 2007;25:3259-65. [Crossref] [PubMed]
- Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015;16:47-56. [Crossref] [PubMed]
- Metz G, Snook K, Sood S, et al. Breast Radiotherapy after Oncoplastic Surgery-A Multidisciplinary Approach. Cancers (Basel) 2022;14:1685. [Crossref] [PubMed]
- Coles CE, Wilson CB, Cumming J, et al. Titanium clip placement to allow accurate tumour bed localisation following breast conserving surgery: audit on behalf of the IMPORT Trial Management Group. Eur J Surg Oncol 2009;35:578-82. [Crossref] [PubMed]
Cite this article as: Heeling E, van Hemert AKE, Vrancken Peeters MJTFD. A clinical perspective on oncoplastic breast conserving surgery. Transl Breast Cancer Res 2023;4:29.


