
CDK4/6 inhibitors for primary endocrine resistant HR-positive/HER2-negative metastatic breast cancer: a case report
Highlight box
Key findings
• Cyclin-dependent kinase 4/6 (CDK4/6) inhibitor in combination with endocrine therapy achieved promising outcomes in a hormone receptor (HR) positive, human epidermal growth factor receptor-2 (HER2)-negative breast cancer patient with liver metastases.
What is known and what is new?
• The efficacy of CDK4/6 inhibitors for HR-positive advanced breast cancer is known, but this case contributes to present and discuss the correlative key clinical issues in a real world context.
• This case also provides an overview of the treatment progress of patients with HR-positive advanced breast cancer, and provides a reference for precision treatment decisions.
What is the implication, and what should change now?
• The good management of this case underscores the crucial role of CDK4/6 inhibitors in HR-positive breast cancer. With the expansion of CDK4/6 inhibitors indications, many patients may be suitable for this therapy regimen earlier, and the use of genetic testing may help with more precise treatment decision.
Introduction
Hormone receptor (HR) positive, human epidermal growth factor receptor-2 (HER2) negative breast cancer accounts for approximately 68% of all early-stage breast cancers (1). Adjuvant endocrine therapy significantly reduces the recurrence rate and mortality in this subset of patients (2). However, about 30% of patients may develop primary endocrine resistant, and about 30% of patients who are effective at initial treatment may develop secondary resistance within 10 years (3), leading to treatment failure. According to European Society for Medical Oncology (ESMO) guidelines, primary endocrine resistance is defined as a relapse less than 2 years after finishing adjuvant endocrine therapy or progression of disease within 6 months on endocrine therapy in metastatic setting. And acquired/acquired resistance is defined as a relapse less than 12 months after finishing endocrine therapy or progression later than 6 months on endocrine therapy for metastatic disease (4). So, optimization of adjuvant therapy strategies and overcoming endocrine resistance have become the focus of clinical practice.
Cyclin-dependent kinase 4 (CDK4) and CDK6 are important for the initiation, growth and survival by mediating cellular transition into S phase of many cancer types (5). CDK4/6 inhibitors are kind of antitumor compounds designed by targeting the adenosinetriphosphate (ATP)-binding domains of the cyclins (6). Chinese Society of Clinical Oncology Breast Cancer (CSCO BC) guidelines in 2021, recommend CDK4/6 inhibitors for adjuvant intensive therapy in patients with high risk clinical and/or pathological features, and in combination with aromatase inhibitor (AI) or fulvestrant (FUL) for standard first-line therapy in metastatic HR-positive breast cancer (7). Here, we report a typical HR-positive, HER2-negative breast cancer patient who received endocrine therapy in combination with a CDK4/6 inhibitor after primary endocrine resistance had achieved one year of progression-free survival. We present this article in accordance with the CARE reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-27/rc).
Case presentation
The patient was diagnosed with left breast invasive breast cancer (cT4bN2M0, stage IIIB, Luminal B HER2-negative type) at the West China Hospital of Sichuan University in November 2020. The patient was diagnosed at age 60 and menopausal at age 53. Denial of family history. Biopsy of left breast revealed invasive ductal carcinoma, immunohistochemistry showed a tumor staining positive for estrogen receptors (80%) and progesterone receptors (20%). HER2 was negative (1+). Ki-67 was 30–40%. Left axillary lymph node metastasis was identified by fine-needle puncture biopsy. Neoadjuvant therapy was used 6 cycles and the protocol was TEC: docetaxel (75 mg/m2, d1), epirubicin (75 mg/m2, d1), and cyclophosphamide (500 mg/m2, d1) intravenous injection every 3 weeks. The efficacy of neoadjuvant treatment was evaluated every 2 cycles according to the Response Evaluation Criteria in Solid Tumors (RECIST1.1). The best response after six cycles of TEC neoadjuvant chemotherapy was partial response (PR) (Figure 1). The patient underwent the modified radical mastectomy with axillary lymph node dissection in May 2021. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
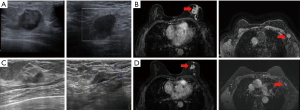
The postoperative pathological stage was ypT2N1M0, stage IIB. The molecular subtypes were Luminal B, HER2-negative. The immunohistochemistry showed a tumor staining positive for estrogen receptors (>95%) and progesterone receptors (2%). HER2 was indeterminate positivity (2+). Ki-67 was 20%. Florescence in situ hybridization (FISH) for HER2 indicates non-amplified. The tumor had a Miller-Payne grade of 2, histological grade of 3, and presented with lymphovascular invasion (LVI). After surgery, patients received radiotherapy to the chest wall and left subclavicular region to doses of 50 Gy/25 f [planning clinical target volume (PCTV)]. Radiotherapy was performed by combination of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT). At the same time received endocrine therapy: letrozole 1 mg daily. In February 2022, reexamination of upper abdominal computed tomography (CT) revealed multiple liver space occupying, no abdominal pain, abdominal distension, jaundice and other symptoms, and liver function testing was normal. Biopsy pathology of liver lesions suggested breast origin. The immunohistochemistry showed a tumor staining positive for estrogen receptors (90%) and progesterone receptors (30–40%). HER2 was negative (1+). Ki-67 was 50%. The disease-free survival (DFS) of AI endocrine therapy was 8 months. Patients received FUL (500 mg d1, d15, d29, then every 28 days after, intramuscular injection) + abesilide (150 mg oral bid) from February 2022 to March 2023. During treatment, the patient developed 1–2 grade diarrhea, which improved after oral administration of montmorillonite powder and imodium. Based on RECIST 1.1, best outcome stable disease (SD) (Figure 2), 12 months progression-free survival (PFS) as of the last follow-up, February 2023.
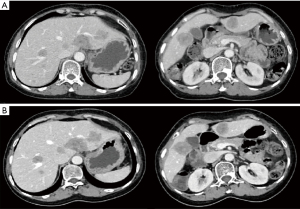
Discussion
This case is typical patient with Luminal B HER2-negative invasive breast cancer. The treatment process (Figure 3) has three important nodes: neoadjuvant, adjuvant, and late-stage first-line treatment decisions. According to American Joint Committee on Cancer (AJCC) staging, the patient is locally advanced disease, and is suitable for neoadjuvant therapy to achieve the descending stage and obtain the opportunity for surgery. For Luminal B HER2-negative invasive breast cancer, neoadjuvant endocrine therapy can be selected as neoadjuvant therapy. However, it is mainly used in highly selective population (hormone-dependent patients who need preoperative treatment but are not suitable for chemotherapy, temporarily not suitable for surgery, or do not need immediate surgery), and is not routinely recommended in clinical practice (7).
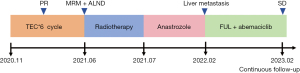
The patient did not achieve complete pathological response (pCR) after neoadjuvant chemotherapy. And the tumor had a Miller-Payne grade of 2. According to monarchE study, abemaciclib (CDK4/6 inhibitor) combined with endocrine therapy (ET) can improvement in invasive disease-free survival (iDFS) in patients with HR+, HER2− node-positive early breast cancer (EBC) at high risk of early recurrence. About 36.5% of the subjects included in this study were non-pCR patients receiving neoadjuvant chemotherapy (8). The early indication for abemaciclib was approved in China in December 2021. The patient received modified radical mastectomy in May 2021, so only prescribed AIs for adjuvant endocrine therapy. On the other hand, the OlympiA study (9) observed a survival benefit of adjuvant olaparib intensive therapy in a subgroup of patients with HR+/HER2− germline breast cancer susceptibility gene (gBRCA) mutations post-neoadjuvant chemotherapy [3-year iDFS 87.5% vs. 78.3%, hazard ratio =0.52 (0.25, 1.04)]. Therefore, breast cancer susceptibility gene testing can be recommended for HR+/HER2− locally advanced breast cancer patients to determine whether there is an opportunity for poly-adenosine diphosphate (ADP)-ribose polymerases (PARPs) inhibitor intensive therapy.
According to the results of clinical studies of MONALEESA-3, PALOMA-3 and MONARCH 2 (10-12), the combined use of CDK4/6 inhibitors in advanced breast cancer patients with primary endocrine resistance can improve the median DFS (mDFS) compared with single drug endocrine therapy. For example, in the MONARCH 2 study, CDK4/6 inhibitors combined with fulvestrant significantly increased median DFS and overall survival (OS) of patients with primary endocrine resistance compared with fulvestrant alone [median PFS (mPFS): 16.3 vs. 7.9 months, hazard ratio =0.447; mOS: 38.7 vs. 31.5 months]. Moreover, the addition of CDK4/6 inhibitors reduced the risk of progression in patients with or without visceral metastasis (hazard ratio =0.47) (12). By integrating the relevant clinical trials, two meta-analyses (13,14) found that CDK4/6 inhibitors combined with endocrine therapy significantly improved PFS in advanced/metastatic HR+/HER2− breast cancer patients, despite an increased occurrence of G3–G4 adverse events. They also suggested that combined CDK4/6 inhibitors improved patient survival regardless of menopausal status, site of metastatic, and endocrine resistance type. Therefore, according to the 2021 CSCO BC guidelines, endocrine therapy combined with targeted therapy with CDK4/6 inhibitors is recommended as the first-line standard treatment for advanced HR+/HER2− patients without visceral crisis (7). However, in patient-specific treatment decisions, the benefits and risks of increased CDK4/6 inhibitors therapy need to be considered. In this case, the patient developed visceral metastases less than 12 months after adjuvant endocrine therapy, which belonged to primary drug resistance to endocrine therapy (4). Nevertheless, the regimen of replacing endocrine drugs and combining CDK4/6 inhibitors still provided the patient with PFS for a longer period of time and no serious adverse events were observed. This fully demonstrates the efficacy and tolerability of CDK4/6 inhibitors in HR+/HER2− metastatic breast cancer.
Conclusions
Here, we report a patient with HR+/HER2− breast cancer who developed disease progression after radiotherapy and AI-assisted endocrine therapy in the non-pCR context of neoadjuvant chemotherapy, and subsequently receiving FUL in combination with CDK4/6 inhibitors to achieve 12 months of progression-free survival. Through this clinical case, we further realize that even patients are resistant to a kind of endocrine drug, another endocrine compound combined with CDK4/6 inhibitor targeted therapy strategy can provide disease remission and survival of HR+/HER2− advanced breast cancer patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-27/rc
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-27/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-23-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med 2015;13:195. [Crossref] [PubMed]
- Haque R, Ahmed SA, Inzhakova G, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev 2012;21:1848-55. [Crossref] [PubMed]
- Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021;32:1475-95. [Crossref] [PubMed]
- Goel S, Bergholz JS, Zhao JJ. Targeting CDK4 and CDK6 in cancer. Nat Rev Cancer 2022;22:356-72. [Crossref] [PubMed]
- Asghar U, Witkiewicz AK, Turner NC, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 2015;14:130-46. [Crossref] [PubMed]
- Available online: http://www.csco.org.cn/cn/index.aspx
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020;38:3987-98. [Crossref] [PubMed]
- Geyer CE Jr, Garber JE, Gelber RD, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol 2022;33:1250-68. [Crossref] [PubMed]
- Slamon DJ, Neven P, Chia S, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol 2018;36:2465-72. [Crossref] [PubMed]
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425-39. Erratum in: Lancet Oncol 2016;17:e136; Lancet Oncol 2016;17:e270. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
- Messina C, Messina M, Zanardi E. Risks and benefits from CDK inhibitors for advanced HR+ Her 2- breast cancer. Ann Oncol 2017;28:3099-100. [Crossref] [PubMed]
- Messina C, Cattrini C, Buzzatti G, et al. CDK4/6 inhibitors in advanced hormone receptor-positive/HER2-negative breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat 2018;172:9-21. [Crossref] [PubMed]
Cite this article as: Luo T, Zhu K, Zhong X, He P, Yan X, Tian T. CDK4/6 inhibitors for primary endocrine resistant HR-positive/HER2-negative metastatic breast cancer: a case report. Transl Breast Cancer Res 2023;4:33.


