
Navigating next-generation HR+/HER2− metastatic breast cancer therapies: a critical commentary on abemaciclib vs. tucidinostat after palbociclib progression
First-line endocrine therapy (ET) in hormone receptor-positive HER2-negative (HR+/HER2−) metastatic breast cancer (MBC) is now combined with any of the three cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i). Under certain conditions, starting this combination in second-line following progression on single agent ET can be an option, as recently reported in the SONIA trial (1-3). Overall, CDK4/6i exhibits a relatively safe tolerability profile, but close monitoring, along with symptom-driven dose adjustments, is crucial to enhance compliance, especially during the first therapy months. Disease progression inevitably occurs following resistance to the combination of ET and CDK4/6i when tumors switch to alternative growth pathways due to existing or acquired mutational changes in genes such as RB, PIK3CA, or ESR1. Phenotypically, they can also lose ER expression or even overexpress HER2, the reason why guidelines advocate for re-biopsy at this stage (4). At progression after CDK4/6i, physicians frequently shift to classic chemotherapy regimens unless there is a low tumor burden or long-term disease control on the prior therapy line. The rationale for this treatment preference lies in the reduction of the median progression-free survival (mPFS) with single-agent ET, such as fulvestrant, from 7 to 2 months following resistance to CDK4/6i treatment.
There remain, however, several treatment options to delay classical chemotherapy. Unfortunately, little is known about the optimal approach as different molecular resistance mechanisms might exist not only against the ET + CDK4/6i combination but sometimes only either against the ET or against the CDK4/6i (Figure 1). In this setting, data testing an early switch to fulvestrant (continuing the CDK4/6i) at the pre-progression appearance of an ESR1 mutation in ctDNA (PADA-1 data; currently being tested in a phase 3 study called SERENA6 NCT04964934), or a switch at progression using single agent elacestrant or camizestrant, both oral selective ER degraders (SERDs), is promising (8). As compared to the standard of care they increased the mPFS in a clinically meaningful way respectively in the EMERALD and SERENA2 trials, especially if ESR1 is mutated or in case of a disease control on the prior CDK4/6i exceeding 12 months (9,10). If ESR1 is not mutated we presume, at this stage, a progressive loss of ER-driven tumor growth. Next-line therapies that targeted the PIK3CA, AKT, or mammalian target of rapamycin (mTOR) pathway (alpelisib, capivasertib, everolimus) improve the mPFS when combined with fulvestrant. A small subset of 20 patients in SOLAR-1 and 121 patients in ByLieve with immediate prior AI + CDK4/6i had benefit when alpelisib was added to fulvestrant respectively tripling mPFS from 1.8 to 5.5 months in SOLAR1 and an mPFS of 7.3 m and clinical benefit rate (CBR) in 50.4% progression-free in ByLieve (11,12). Another trial called CAPITELLO 291 included patients with resistant disease to ET + CDK4/6i combinations. They received fulvestrant +/− capivasertib (a small molecule inhibitor of AKT) (13). As in ByLieve, patients in this trial with a short duration of prior CDK4/6i benefit (<12 months) had a doubling of mPFS (from 2 to 4.9 months) in the capivasertib arm. The combination fulvestrant-everolimus (an mTOR inhibitor), tested in the MANTA trial prior to the widespread use of CDK4/6i suggested that this combo is a safe option to consider for patients post CDK4/6i progression (14). Large clinical data on its efficacy post ET + CDK4/6i progression in HR+/HER2− MBC is lacking. The largest study in this setting by Dhakal et al. showed that everolimus-ET combinations in 41 women were associated with mPFS of 4.2 months and objective response rate (ORR) of 17.1% (15).
A subset of patients resistant to the combination might be resistant to ET or CDK4/6i only and might derive clinical benefit from continued CDK4/6 inhibition combined with another type of ET. It turns out this is only if the CDK4/6i was changed as shown in real-world datasets by Martin et al. and with ribociclib following progression on palbociclib in the small phase 2 trial MAINTAIN, doubling mPFS [hazard ratio =0.57; confidence interval (CI) (0.39–0.85)] (16,17). This was not observed in PACE nor in PALMIRA where palbociclib was maintained and only the endocrine partner was changed (18,19). Another approach is continuous triplet therapy combining ET and CDK4/6i with for example an mTOR inhibitor. TRINTI-1 phase I/II trial enrolled 104 patients with CDK4/6i + ET-refractory HR+/HER2– advanced breast cancer testing ribociclib with exemestane and everolimus (20). It was tolerable with a CBR of 41.1% at week 24 exceeding the predefined threshold as the primary endpoint in this study. However, drug-drug interaction observed between everolimus and ribociclib precluded further exploration. Other ongoing studies like INAVO (Inavolisib; NCT05646862) and CAPITELLO 292 (Capivasertib; NCT04862663) are testing triple therapy with an ET, a CDK4/6i and an inhibitor of PIK3CA or AKT respectively.
Following ET + CDK4/6i progression, limited data compared standard treatments with chemotherapy (Figure 2). Cogliati et al. reviewed these experiences and concluded that continuing CDK4/6i or switching to another CDK4/6i can remain effective post-progression (22). Additionally, ‘chemotherapy-like’ treatments like antibody-drug conjugates (ADCs) such as trastuzumab-deruxtecan (T-DXd) and sacituzumab-govitecan (SG) expand treatment options for HR-resistant and HER2− low/negative MBC (23,24). T-DXd, which combines a HER2-targeting antibody and a topoisomerase I inhibitor, has shown substantial responses and improved overall survival (OS) in these patient populations. DESTINY-Breast04 compared T-DXd to the physician’s choice of chemotherapy in HER2− low MBC. It not only showed a longer mPFS in T-DXd users (10.1 vs. 5.4 months) but also a longer OS (23.9 vs. 17.5 months) in the HR+ cohort (25). Accurate measurement of HER2 expression is crucial as T-DXd benefits patients with low HER2 expression.
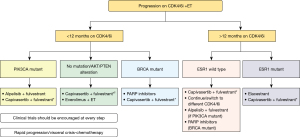
SG is another type of ADC consisting of a humanized anti-Trop-2 monoclonal antibody linked to SN-38, an active metabolite of irinotecan, also tested in triple-negative tumors. It has also shown notable response rates and survival benefits in HR+/HER2− MBC, particularly after multiple prior therapies (25). SG demonstrated a statistically significant improvement in OS vs. Treatment of Physician’s Choice with a median survival benefit of 3.2 months (26). SG has a manageable safety profile with a low incidence of treatment discontinuation due to adverse events (AEs), mainly neutropenia and diarrhea. However, both T-DXd and SG, while less toxic than traditional chemotherapy, can still have severe toxicities and are costly. Also, they are not yet widely available in many countries. Overall, the choice of therapy after CDK4/6i progression depends on several factors, but the duration of disease control achieved during the previous therapy line is crucial. Ongoing research aims to identify additional biomarkers for refined patient selection in this challenging group.
In this endocrine-resistant setting of progression post CDK4/6 inhibition, the study by Yuan et al. assumes critical significance (26). It augments our comprehension of the real-world clinical outcomes associated with the administration of oral subtype-selective histone deacetylase (HDAC) inhibitor-based therapy subsequent to CDK4/6i, especially following palbociclib usage. This retrospective cohort study, utilizing data from the Breast Cancer Database of the Chinese Society of Clinical Oncology, aimed to compare the therapeutic efficacy of tucidinostat-based treatments (76 patients) with those receiving abemaciclib-based treatments (73 patients) in metastatic HR+/HER2− breast cancer. Notably, direct comparisons were constrained by the inherent heterogeneity in this patient population and the differing proportions of patients receiving abemaciclib as a subsequent treatment after CDK4/6i resistance (50% for abemaciclib vs. 30% for tucidinostat). The study revealed less favorable progression-free survival in the HDAC inhibitor-treated group (mPFS of 2 months), as compared to the abemaciclib-treated group (mPFS of 5 months). Additionally, among abemaciclib-treated patients, those with PIK3CA wild-type status showed even more promising outcomes, as indicated by subset analysis with available next-generation sequencing (NGS) data. It is pertinent to mention that preliminary data from a subset of patients in the Yuan et al. study (n=44) had previously been disclosed, encompassing a median follow-up duration of 10 months (ranging from 1 to 26 months), with a data cutoff date in February 2022. This preliminary dataset reported a CBR of 6.8% (3/44), an mPFS of 2.0 months (95% CI: 1.9–2.1), and a median overall survival (mOS) of 14 months (95% CI: 6.3–21.7). Moreover, the mPFS was notably extended to 4.1 months (95% CI: 0–8.2) in patients presenting with solitary metastatic sites, while those who received tucidinostat following CDK4/6i failure exhibited an mPFS of 4.5 months (95% CI: 4.2–4.8). Multivariate analysis findings suggested that patients with a solitary metastatic site or those receiving sequential tucidinostat therapy after CDK4/6i failure were more likely to derive clinical benefit from tucidinostat in combination with ET. Furthermore, these preliminary data hinted at a potential association between PIK3CA mutations and resistance to tucidinostat therapy, a notion previously speculated upon by Zhou et al. (27). However, given suboptimal outcomes in various subgroups, including an additional 29 patients, alternative therapies should be considered. The superiority of abemaciclib in later lines aligns with MONARCH 1 study findings, suggesting its potential as an alternative treatment strategy (28). Retrospective case series of abemaciclib post-palbociclib are promising and showed that abemaciclib has distinct pharmacokinetic and pharmacodynamic properties (1). Currently, results from large phase 3 trials of abemaciclib after CDK4/6i are not yet available (the ongoing phase 3 EMBER3 NCT04975308 and the POSTMONARCH trial NCT05169567). Molecular predictors of cross-resistance to CDK4/6i therapy are being explored. The development of a predictive gene panel for activity of abemaciclib after palbociclib progression in patients with an ESR1-mutated MBC is promising (29). However, challenges related to the availability, price, and toxicity of these targeted agents remain a concern for both patients and clinicians.
HDAC are key epigenetic modifiers known for restoring estrogen-receptor dependency in endocrine-resistant cases. While much of this knowledge stems from preclinical studies, HDAC inhibitors’ clinical history predating CDK4/6i is mixed. Tucidinostat, approved in China and used in other cancers, showed safety (no grade 4 AEs, treatment-related deaths, 9.1% dose reductions) and efficacy promise by doubling progression-free survival when combined with exemestane in the ACE trial (30). It might be an option, particularly for patients with lower tumor burdens and limited prior palliative treatments. In contrast, performance using entinostat, another HDAC inhibitor, was disappointing and lacks Food and Drug Administration (FDA)/European Medicines Agency (EMA) approval (31).
In the E2112 trial, researchers randomized 608 patients with HR+/HER2− MBC (median age 63 years) to exemestane +/− entinostat between March 2014 and October 2018: 35% had prior CDK4/6i therapy (32). Grade 3 and 4 AEs in the exemestane + entinostat arm included neutropenia, hypophosphatemia, anemia, leukopenia, fatigue, diarrhea, and thrombocytopenia. There was no significant difference in mPFS or mOS between the two arms (mPFS: 3.3 vs. 3.1 months; mOS: 23.4 vs. 21.7 months; hazard ratio =0.99; 95% CI: 0.82–1.21; P=0.94). The ORR was 5.8% in the exemestane plus entinostat arm and 5.6% in the exemestane plus placebo arm.
The optimal therapeutic strategy post-CDK4/6i progression remains an open question, necessitating ongoing research into molecular biomarkers that can predict treatment efficacy and provide a deeper understanding of resistance mechanisms to CDK4/6i. Additionally, differences in toxicity profiles among novel agents may lead to personalized therapeutic strategies for clinical practice. The current incorporation of adjuvant CDK4/6i in the management of high-risk HR+/HER2− breast cancer challenges re-exposure at metastatic relapse. These agents in the adjuvant setting not only prevent or delay recurrence but might also reshape the tumor’s biology with an impact on the selection of subsequent therapies. This impact doesn’t necessarily exclude the continued use of CDK4/6i unless specific pathway alterations must be targeted. Key factors influencing the choice of first-line metastatic therapy post-adjuvant CDK4/6i progression include the extent and localization of the tumor, primary clonal or acquired molecular events, and duration to metastasis (disease-free interval) or duration of prior disease control (33,34). This calls for ongoing research and personalized approaches to optimize outcomes underscoring the pivotal role played by real-world registries, such as PRAEGNANT, in shedding light on the adherence, tolerance, and efficacy of treatment modalities across a broad patient population, surpassing the constraints of clinical trials (35). The pertinence of real-world data is poised to grow exponentially in the coming years, given the burgeoning array of emerging treatment choices in HR+/HER2− MBC that may not be comprehensively evaluated through traditional clinical investigations.
Acknowledgments
We would like to acknowledge our colleagues at the University of Leuven (KU Leuven) for their valuable insights and contributions throughout the research process.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Breast Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-41/coif). HW reports that his institution received financial compensation on his behalf for advisory boards, lecture fees, and/or consultancy fees from Daiichi Sankyo, Gilead, Lilly, Augustine Therapeutics, AstraZeneca, Immutep Pty, MSD and Roche. He received travel support from Gilead, Daiichi Sankyo and Pfizer. PN reports that his institution received financial compensation on his behalf for advisory boards, lecture fees, and/or consultancy fees from Pfizer, Novartis, AstraZeneca, Minarini, Roche and Gilead. He received travel support from Novartis, AstraZeneca, Pfizer, Roche and Eli Lilly. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnston S, Emde A, Barrios C, et al. Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors: existing and emerging differences. JNCI Cancer Spectr 2023;7:pkad045.
- Sonke GS, Van Ommen-Nijhof A, Wortelboer N, et al. Primary outcome analysis of the phase 3 SONIA trial (BOOG 2017-03) on selecting the optimal position of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors for patients with hormone receptor-positive (HR+), HER2-negative (HER2-) advanced breast cancer (ABC). J Clin Oncol 2023;41:LBA1000. [Crossref]
- Burstein HJ, Somerfield MR, Barton DL, et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: ASCO Guideline Update. J Clin Oncol 2021;39:3959-77. [Crossref] [PubMed]
- Henry NL, Somerfield MR, Dayao Z, et al. Biomarkers for Systemic Therapy in Metastatic Breast Cancer: ASCO Guideline Update. J Clin Oncol 2022;40:3205-21. [Crossref] [PubMed]
- Fan W, Chang J, Fu P. Endocrine therapy resistance in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem 2015;7:1511-9. [Crossref] [PubMed]
- Yang J, Nie J, Ma X, et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. [Crossref] [PubMed]
- Ji X, Lu Y, Tian H, et al. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother 2019;114:108800. [Crossref] [PubMed]
- Bidard FC, Hardy-Bessard AC, Dalenc F, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2022;23:1367-77. [Crossref] [PubMed]
- SABCS 2022: EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2- metastatic breast cancer: Updated results by duration of prior CDK4/6i in metastatic setting. Accessed September 11, 2023. Available online: https://clin.larvol.com/abstract-detail/SABCS%202022/60535042
- SABCS 2022: Camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: Results of the randomized, multi-dose Phase 2 SERENA-2 trial. Accessed September 11, 2023. Available online: https://clin.larvol.com/abstract-detail/SABCS%202022/61701459
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol 2021;22:489-98. [Crossref] [PubMed]
- André F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol 2021;32:208-17. [Crossref] [PubMed]
- Turner NC, Oliveira M, Howell SJ, et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2023;388:2058-70. [Crossref] [PubMed]
- Schmid P, Zaiss M, Harper-Wynne C, et al. Fulvestrant Plus Vistusertib vs Fulvestrant Plus Everolimus vs Fulvestrant Alone for Women With Hormone Receptor-Positive Metastatic Breast Cancer: The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1556-64. [Crossref] [PubMed]
- Dhakal A, Antony Thomas R, Levine EG, et al. Outcome of Everolimus-Based Therapy in Hormone-Receptor-Positive Metastatic Breast Cancer Patients After Progression on Palbociclib. Breast Cancer (Auckl) 2020;14:1178223420944864. [Crossref] [PubMed]
- Martin JM, Handorf EA, Montero AJ, et al. Systemic Therapies Following Progression on First-line CDK4/6-inhibitor Treatment: Analysis of Real-world Data. Oncologist 2022;27:441-6. [Crossref] [PubMed]
- Kalinsky K, Accordino MK, Chiuzan C, et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin-dependent kinase 4/6 inhibition (CDK 4/6i) in patients (pts) with unresectable or hormone receptor–positive (HR+), HER2-negative metastatic breast cancer (MBC): MAINTAIN trial. J Clin Oncol 2023;41:4004-13. [Crossref] [PubMed]
- SABCS 2022: Palbociclib After CDK4/6i and Endocrine Therapy (PACE): A Randomized Phase II Study of Fulvestrant, Palbociclib, and Avelumab for Endocrine Pre-treated ER+/HER2- Metastatic Breast Cancer. Accessed September 9, 2023. Available online: https://clin.larvol.com/abstract-detail/SABCS%202022/60535046
- Llombart-Cussac A, Harper-Wynne C, Perello A, et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR[+])/human epidermal growth factor receptor 2-negative (HER2[-]) advanced breast cancer (ABC): PALMIRA trial. J Clin Oncol 2023;41:1001. [Crossref]
- Bardia A, Hurvitz SA, DeMichele A, et al. Phase I/II Trial of Exemestane, Ribociclib, and Everolimus in Women with HR(+)/HER2(-) Advanced Breast Cancer after Progression on CDK4/6 Inhibitors (TRINITI-1). Clin Cancer Res 2021;27:4177-85. [Crossref] [PubMed]
- Mittal A, Molto Valiente C, et al. Filling the Gap after CDK4/6 Inhibitors: Novel Endocrine and Biologic Treatment Options for Metastatic Hormone Receptor Positive Breast Cancer. Cancers (Basel) 2023;15:2015. [Crossref] [PubMed]
- Cogliati V, Capici S, Pepe FF, et al. How to Treat HR+/HER2- Metastatic Breast Cancer Patients after CDK4/6 Inhibitors: An Unfinished Story. Life (Basel) 2022;12:378. [Crossref] [PubMed]
- Mosele F, Deluche E, Lusque A, et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat Med 2023;29:2110-20. [Crossref] [PubMed]
- Rugo HS, Bardia A, Marmé F, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol 2022;40:3365-76. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Yuan Y, Zhang S, Wang T, et al. Efficacy and safety of abemaciclib-based therapy versus tucidinostat-based therapy after progression on palbociclib in patients with HR+HER2− metastatic breast cancer. Transl Breast Cancer Res 2023;4:10. [Crossref]
- Zhou J, Wu X, Zhang H, et al. Clinical outcomes of tucidinostat-based therapy after prior CDK4/6 inhibitor progression in hormone receptor-positive heavily pretreated metastatic breast cancer. Breast 2022;66:255-61. [Crossref] [PubMed]
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(-) Metastatic Breast Cancer. Clin Cancer Res 2017;23:5218-24. [Crossref] [PubMed]
- Brett JO, Dubash TD, Johnson GN, et al. A Gene Panel Associated With Abemaciclib Utility in ESR1-Mutated Breast Cancer After Prior Cyclin-Dependent Kinase 4/6-Inhibitor Progression. JCO Precis Oncol 2023;7:e2200532. [Crossref] [PubMed]
- Jiang Z, Li W, Hu X, et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:806-15. [Crossref] [PubMed]
- Sun Y, Hong JH, Ning Z, et al. Therapeutic potential of tucidinostat, a subtype-selective HDAC inhibitor, in cancer treatment. Front Pharmacol 2022;13:932914. [Crossref] [PubMed]
- Connolly RM, Zhao F, Miller KD, et al. E2112: Randomized Phase III Trial of Endocrine Therapy Plus Entinostat or Placebo in Hormone Receptor-Positive Advanced Breast Cancer. A Trial of the ECOG-ACRIN Cancer Research Group. J Clin Oncol 2021;39:3171-81. [Crossref] [PubMed]
- Johnston SRD, Toi M, O'Shaughnessy J, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol 2023;24:77-90. [Crossref] [PubMed]
- Slamon DJ, Fasching PA, Hurvitz S, et al. Rationale and trial design of NATALEE: a Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breast cancer. Ther Adv Med Oncol 2023;15:17588359231178125. [Crossref] [PubMed]
- Lux MP, Nabieva N, Hartkopf AD, et al. Therapy Landscape in Patients with Metastatic HER2-Positive Breast Cancer: Data from the PRAEGNANT Real-World Breast Cancer Registry. Cancers (Basel) 2018;11:10. [Crossref] [PubMed]
Cite this article as: Neven P, Dullens L, Han S, Deblander A, Van Herck Y, Van Houdt M, Wildiers H. Navigating next-generation HR+/HER2− metastatic breast cancer therapies: a critical commentary on abemaciclib vs. tucidinostat after palbociclib progression. Transl Breast Cancer Res 2023;4:31.


