
Poland syndrome combined with breast cancer: a case report
Highlight box
Key findings
• The anatomic abnormality of Poland syndrome has some influence on the diagnosis and treatment of breast cancer, especially in the choice of surgical mode.
What is known and what is new?
• An association between Poland syndrome and breast cancer has been reported.
• The anatomic variation of patients with Poland syndrome has some influence on the selection of surgical methods for breast cancer, but whether it will affect the prognosis of patients is unknown.
What is the implication, and what should change now?
• Surgeons need to consider more carefully and comprehensively in the selection of surgical methods for patients with Poland syndrome combined with breast cancer. To clarify the link between Poland syndrome and breast cancer, we need more cases for etiological studies.
Introduction
Poland syndrome is an occasional congenital malformation characterized by unilateral chest wall dysplasia and ipsilateral upper limb abnormalities (1). Typical clinical features include the partial absence of pectoralis major muscle, costosternum, pectoralis minor muscle, breast or nipple hypoplasia or absence, hypoplasia of subcutaneous tissue, funnel malformation and rib regeneration disorders, and absence of costal cartilage or ribs two, three, four or three, four and five. Chest wall defect is often accompanied by a pulmonary hernia. Its clinical manifestations are extremely variable and it is rare to identify all features in a single individual. Most of these anatomical defects involve only one side of the body, while the right side is more common (2). Bilateral dysplasia is extremely rare but has been reported (1). The etiology of Poland syndrome is currently inconclusive, but it is generally thought to be caused by developmental abnormalities associated with the subclavian artery and branch vessels during the critical 6th week of embryonic development (3), which results in decreased perfusion on the affected side of the chest wall. The association of Poland syndrome with various malignancies has been reported, including lung cancer (4), leiomyosarcoma (5), leukemia (6), lymphoma (7), Wilms tumor (8), neuroblastoma (9) and breast cancer (10-12). The anatomical abnormalities of Poland syndrome may affect the treatment of breast cancer. We present this case in accordance with the CARE reporting checklist (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-46/rc).
Case presentation
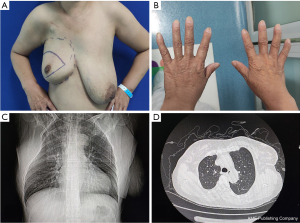
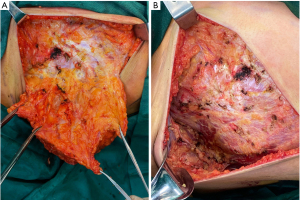
The patient reported was a 47-year-old female admitted to the Department of General Surgery, the First Medical Center of the Chinese People’s Liberation Army (PLA) General Hospital on February 7th, 2022. Prior to admission, the patient underwent a breast ultrasound examination at a local hospital on January 19th, 2022, and the results showed: strong echogenic nodules in the left breast, American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) 4a; low echogenic nodules in the right breast, BI-RADS 4c, enlarged lymph nodes in the right armpit. A biopsy of the right breast mass and right axillary abnormal lymph node was performed. The pathological results showed that the right breast mass was breast invasive carcinoma, non-specific type, World Health Organization (WHO) II grade. The lymphoid tissue in the right axilla was reactive hyperplasia, and no metastatic tumor was found. The puncture tissue of the left breast showed chronic inflammatory changes. The pathological consultation of the First Medical Center of the Chinese PLA General Hospital reported that the immunohistochemical results were estrogen receptor (ER) (−), progesterone receptor (PR) (−), HER-2 (3+), p53 (+), Ki-67 (+ 25–50%). Luminal type: HER-2 overexpression. Physical examination: Figure 1 shows the physical and imaging examinations of the patient. The patient’s bilateral mammary glands were obviously asymmetrical, and the right mammary gland was hypoplasia, the tumor did not involve the skin (Figure 1A). A tumor measuring 4 cm × 5 cm was palpated in the upper outer quadrant of the right breast, with short fingers on the right hand without syndactyly (Figure 1B), and no other obvious abnormalities were observed. Auxiliary examination after admission: breast ultrasound examination revealed hypoechoic nodules in the right breast, BI-RADS 6, and the tumor size was 3.9 cm × 2.8 cm × 4.6 cm; and enlarged lymph nodes in the right armpit. The clinical tumor-node-metastasis (TNM) classification was cT2N0M0. Chest X-ray showed multiple rib malformations on the right side (Figure 1C). Chest computed tomography (CT) indicated thoracic asymmetry, bone deficiency in the right anterior ribs of the third and fourth, the right pectoralis major and pectoralis minor were all missing, no abnormalities in thoracic bone structure and soft tissue, and no enlargement in both pulmonary hilus (Figure 1D). The patient self-reported that the right anatomical abnormalities caused by Poland syndrome did not significantly affect her daily life and there was no family history of Poland syndrome. Considering the patient’s tumor size, immunohistochemical results, and other conditions, the decision was made to give the patient neoadjuvant therapy first. The patient was enrolled in the clinical research program and had signed the relevant informed consent. Starting on February 15th, 2022, patients were given four cycles of epirubicin (Pfizer) 120 mg plus cyclophosphamide 800 mg, D1 plus pyrotinib (400 mg, once daily) followed by four cycles of docetaxel (Qilu) 120 mg plus trastuzumab (Hufuhong) 420 mg, D1 plus pyrotinib (400 mg, once daily) as a neoadjuvant regimen. Gastrointestinal symptoms such as mild nausea and vomiting occurred during chemotherapy, and the adverse reactions were grade I according to WHO classification, which were improved after symptomatic treatment. No other obvious adverse reactions were observed. On September 5th, 2022, a breast ultrasound reexamination showed that the size of the right breast tumor was 0.7 cm × 0.6 cm × 0.6 cm, and the efficacy was evaluated as partial remission (PR) at the end of chemotherapy. This patient underwent a modified radical mastectomy for right breast cancer on September 7th, 2022, during which she was found to have an absence of the right pectoralis major and minor muscles (Figure 2), along with an adhesion band along the parasternal to the right axillae. The postoperative routine pathological report of the patient was ductal carcinoma in situ in the right breast tumor bed, medium grade, focal with invasion, and the size of the tumor was 2 cm × 1 cm × 1 cm. The breast interstitial tissue around the tumor bed showed hyperplasia with hyalinization, local calcification and lymphocyte infiltration, which were consistent with the changes after treatment. No cancer cells were found in the nipple, skin, or basal incisal margin. No metastatic carcinoma was found in axillary lymph nodes (0/22). The Miller & Payne (MP) Assessment System rating for postoperative pathology was G3. The postoperative pathological classification after neoadjuvant therapy was pT1N0M0. After surgery, the patient continued to receive adjuvant treatment with trastuzumab plus pertuzumab regimen, and the specific dose was: trastuzumab (Hufuhong) 390 mg for 1 year, the first dose of pertuzumab was 840 mg, and the subsequent dose was 420 mg. The patient was followed up for 8 months after the operation, and the function of the affected limb recovered well without complications such as subcutaneous effusion, flap necrosis, upper limb edema, and chronic pain. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
A clear association between developmental malformations and breast tumors has not been established, but it is worth noting the diagnosis and treatment of Poland syndrome in conjunction with breast cancer: (I) although most breast tumors occur in the ipsilateral underdeveloped breast, the ipsilateral normal breast, contralateral breast and reconstructed breast may also be involved (11-15). Therefore, in the breast examination of patients suspected of breast cancer, we should not ignore the possibility of tumor formation on both the underdeveloped and normal sides of the breast. (II) Poland syndrome usually presents with the absence of pectoralis major and pectoralis minor muscles and abnormal anatomical position of lats, resulting in thinning of the chest on the affected side. Therefore, the operator should be more careful and cautious when performing invasive operations such as local anesthesia, fine needle aspiration cytology, and tumor resection to avoid damage to neighboring tissues or organs (16). The anatomic position of the latissimus dorsi can also affect the scope of resection in modified radical mastectomy because the anterior latissimus dorsi is an important anatomic marker of the scope of resection in modified radical mastectomy. (III) Patients with Poland syndrome combined with breast cancer may be more prone to lymph node metastasis, so when breast cancer tumor resection is performed, axillary lymph node should be accurately evaluated and the scope of lymph node dissection should be reasonably defined (17). (IV) Poland syndrome also has an impact on breast reconstruction. The main methods of breast reconstruction are divided into autologous tissue breast reconstruction and implant breast reconstruction, the latter is still the most commonly used at home and abroad. Implant breast reconstruction can be divided into prepectoral breast reconstruction (PBR) and subpectoral breast reconstruction (SBR) according to the anatomical location of implant placement (18). SBR is the traditional method of inserting implants behind the pectoralis major muscle, including full muscle coverage with pectoralis major and serratus anterior muscle, and partial muscle coverage with acellular dermal matrix (ADM) or synthetic mesh. The coverage of the pectoral muscle can increase the safety of the operation and the concealment of the implant, but the adverse reactions caused by the dissection of the chest wall will also be brought. Because of the absence of the pectoralis major and minor muscles and chest wall malformations in patients with Poland syndrome, SBR often cannot be performed. In PBR, the implant is directly implanted into the native breast anatomical space between the flap and the pectoralis major muscle which has the advantages of small trauma, light pain, avoiding movement deformity, natural breast ptosis, relatively easy operation, and short learning curve. However, there are also limitations, including the lack of sufficient soft tissue coverage, which may easily lead to poor aesthetics such as obvious contour and ripple sign of the implant, and complications of the flap or incision may easily lead to reconstruction failure. In recent years, with the common development and progress of mastectomy technology, mesh materials, implant technology, tissue perfusion monitoring, autologous fat transplantation, and other fields, the application of PBR has been promoted, and it has shown a rapid increase trend. PBR has similar surgical safety to SBR and has advantages over SBR in reducing postoperative pain, eliminating motor deformity, reducing cyst contracture, and improving patient satisfaction (19), which is also the reason why PBR is becoming increasingly popular. On the premise of ensuring the safe resection of the tumor, obtaining a flap with a certain thickness and good blood perfusion is a necessary condition for PBR surgery (20), so more caution should be taken in the selection of patients. However, in patients with Poland syndrome, the skin flap on the affected side is usually thin, so it may be more prone to complications such as postoperative flap ischemia and poor wound healing, resulting in failure of reconstruction. In summary, the choice of implant breast reconstruction in patients with Poland syndrome is limited, the reconstruction is more difficult, and there may be a higher incidence of postoperative complications. (V) Absence of pectoralis major or/and pectoralis minor muscles also has an impact on patients receiving radiotherapy. Postoperative adjuvant radiotherapy is the standard treatment for breast cancer patients who have undergone breast-conserving surgery. Patients with stage T3 to 4 or with positive axillary lymph nodes also need radiation therapy after mastectomy. Normally, the associated lung exposure during breast radiation therapy is low, and radiation-induced lung injuries are rare (21). During single breast radiotherapy, radiation-induced pneumonitis occurs in the peripheral lung or in the apical region of the lung if segmental radiotherapy is performed (22). However, Poland syndrome patients with defects such as loss of pectoralis major and minor muscles and hypoplasia of the chest wall may increase the incidence of complications such as radiation-induced lung injury and post-radiation cardiovascular toxicity if radiotherapy is required after breast cancer surgery, but there is a lack of research to confirm it. Taking all these factors into consideration, we performed a modified radical mastectomy of the right breast. Therefore, surgeons need to consider more carefully and comprehensively in the selection of surgical methods for patients with Poland syndrome combined with breast cancer.
Conclusions
The pathogenesis of Poland syndrome is currently inconclusive and its association with breast cancer needs further study. However, the anatomic abnormality of Poland syndrome has some influence on the diagnosis and treatment of breast cancer. In order to clarify the link between Poland syndrome and breast cancer, we need more cases for etiological studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-46/rc
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-46/prf
Conflicts of Interest: All authors have completed the ICMJE unified disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-46/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- DeFazio MV, Dervishaj OA, Bozzuto LM, et al. Delayed Recurrent and Bilateral Breast Cancer in Patients With Partial Poland's Anomaly: Report of 2 Rare Cases and Review of the Literature. Clin Breast Cancer 2018;18:e285-90. [Crossref] [PubMed]
- Tafti D, Cecava ND. Poland Syndrome. 2023 May 22. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
- Nakagawa T, Oda G, Kato R, et al. A Case of Breast Cancer in a Patient with a Congenital Pectoralis Muscle Defect. Case Rep Oncol 2021;14:1092-6. [Crossref] [PubMed]
- Ahn MI, Park SH, Park YH. Poland's syndrome with lung cancer. A case report. Acta Radiol 2000;41:432-4. [Crossref] [PubMed]
- Shaham D, Ramu N, Bar-Ziv J. Leiomyosarcoma in Poland's syndrome. A case report. Acta Radiol 1992;33:444-6. [Crossref] [PubMed]
- Boaz D, Mace JW, Gotlin RW. Poland's syndrome and leukaemia. Lancet 1971;1:349-50. [Crossref] [PubMed]
- Sackey K, Odone V, George SL, et al. Poland's syndrome associated with childhood non-Hodgkin's lymphoma. Am J Dis Child 1984;138:600-1. [PubMed]
- Athale UH, Warrier R. Poland's syndrome and Wilms tumor: an unusual association. Med Pediatr Oncol 1998;30:67-8. [Crossref] [PubMed]
- Caksen H, Patiroglu T, Ozdemir MA, et al. Neuroblastoma and Poland syndrome in a 15-year-old boy. Acta Paediatr Jpn 1997;39:701-4. [Crossref] [PubMed]
- Havlik RJ, Sian KU, Wagner JD, et al. Breast cancer in Poland syndrome. Plast Reconstr Surg 1999;104:180-2. [Crossref] [PubMed]
- Katz SC, Hazen A, Colen SR, et al. Poland's syndrome and carcinoma of the breast: a case report. Breast J 2001;7:56-9. [Crossref] [PubMed]
- Okamo H, Miura K, Yamane T, et al. Invasive ductal carcinoma of the breast associated with Poland's syndrome: report of a case. Surg Today 2002;32:257-60. [Crossref] [PubMed]
- Tamiolakis D, Venizelos D, Antoniou C, et al. Breast cancer development in a female with Poland's syndrome. Onkologie 2004;27:569-71. [PubMed]
- DeFazio MV, Graziano FD, Lakhiani C, et al. Incidental discovery of partial Poland's sequence during mastectomy for contralateral breast cancer. Breast J 2019;25:316-7. [Crossref] [PubMed]
- Samuels TH, Haider MA, Kirkbride P. Poland's syndrome: a mammographic presentation. AJR Am J Roentgenol 1996;166:347-8. [Crossref] [PubMed]
- Salhab M, Al Sarakbi W, Perry N, et al. Pneumotho rax after a clinical breast fifine-needle aspiration of a lump in a patient with Poland’s syndrome. Int Semin Surg Oncol 2005;2:14. [Crossref] [PubMed]
- Zhang F, Qi X, Xu Y, et al. Breast cancer and Poland's syndrome: a case report and literature review. Breast J 2011;17:196-200. [Crossref] [PubMed]
- Chopra S, Al-Ishaq Z, Vidya R. The Journey of Prepectoral Breast Reconstruction through Time. World J Plast Surg 2021;10:3-13. [Crossref] [PubMed]
- Balk EM, Earley A, Avendano EA, et al. Long-Term Health Outcomes in Women With Silicone Gel Breast Implants: A Systematic Review. Ann Intern Med 2016;164:164-75. [Crossref] [PubMed]
- Rancati AO, Angrigiani CH, Hammond DC, et al. Direct to Implant Reconstruction in Nipple Sparing Mastectomy: Patient Selection by Preoperative Digital Mammogram. Plast Reconstr Surg Glob Open 2017;5:e1369. [Crossref] [PubMed]
- Chargari C, Riet F, Mazevet M, et al. Complications of thoracic radiotherapy. Presse Med 2013;42:e342-51. [Crossref] [PubMed]
- Neal AJ, Yarnold JR. Estimating the volume of lung irradiated during tangential breast irradiation using the central lung distance. Br J Radiol 1995;68:1004-8. [Crossref] [PubMed]
Cite this article as: Guo S, Chen Y, Li Q, Xie Y, Hao X, Wang J. Poland syndrome combined with breast cancer: a case report. Transl Breast Cancer Res 2024;5:7.


