
Challenges in HER2-low breast cancer identification, detection, and treatment
Introduction
Breast cancer (BC) is a heterogeneous disease that can be classified into 4 molecular subtypes [luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-positive, and triple-negative BC (TNBC)] according to the expression of 4 molecular markers including estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki67 (1). The molecular subtype is closely related to prognosis and also offers a basis for tailored treatment. In particular, HER2-targeted therapies have greatly improved the prognosis of patients with HER2-positive BC (2). The DESTINY-Breast04 (DB-04) phase 3 trial has shown that HER2-low BC patients could also benefit from trastuzumab deruxtecan (T-Dxd), a novel anti-HER2 antibody-drug conjugate (ADC) (3). Therefore, the concept of HER2-low, as an indicator of the population benefiting from T-Dxd treatment, is an important update and challenge to the current classification and treatment of BC and has become a new priority in both clinical practice and scientific research. Further, it has led to the revision and optimization of the current clinical pathways for HER2-negative metastatic BC (MBC). Nevertheless, there are still some concerns and controversies.
Is HER2-low a new subtype?
In the previous clinical pathways, BC could be classified as HER2-positive or HER2-negative according to the expression of HER2.
BC is a heterogeneous disease, with HER2-positive BC representing 13–15% of all breast tumors. The HER2 pathway may become activated by HER2 overexpression on the surface of tumor cells following ERBB2 gene amplification. HER2 belongs to the human epidermal growth factor receptor (HER) family which also includes HER1, HER2, HER3, and HER4. These receptors consist of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. HER2 undergoes hetero- or homo-dimerization upon binding to ligands, leading to phosphorylation of the intracellular domains, which activates downstream signaling pathways, triggers downstream gene transcription, and promotes the proliferation, survival, invasion, and metastasis of tumor cells. Studies have confirmed that HER2 amplification and/or overexpression has a tumor-driving effect in a wide range of tumors, making HER2 both an important biomarker for prognostic prediction and a key therapeutic target. Anti-HER2 therapies have been shown to substantially improve the prognosis of patients with HER2-positive BC.
According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines for HER2 testing in BC, HER2 status is assessed by immunohistochemistry (IHC) and in situ hybridization (ISH) (4). IHC 0 is defined by “no staining observed or membrane staining that is incomplete and is faint/barely perceptible and within ≤10% of the invasive tumor cells”; IHC 1+ is defined by “incomplete membrane staining that is faint/barely perceptible and within >10% of the invasive tumor cells”; IHC 2+ is defined as “circumferential membrane staining that is incomplete and/or weak/moderate and within >10% of the invasive tumor cells, or complete and circumferential membrane staining that is intense and within ≤10% of the invasive tumor cells”; IHC 3+ is defined as “circumferential membrane staining that is intense, complete, and uniform within >10% of invasive cancer cells”. Among them, patients with IHC 2+ are required to further undergo ISH. Currently, in the vast majority of studies, including the phase 3 DB-04 trial, HER2-low BC is defined as BC with a HER2 IHC score of 1+ or 2+ and negative ISH result, and this criterion has also been favored by most experts in the European Society for Medical Oncology (ESMO) expert consensus (5). Early assessments suggests that HER2-low expression BC accounts for approximately 45–55% of all BC cases (6). Furthermore, the proportion of HER2-low is higher in hormone receptor (HR)-positive disease (55–65%) than in TNBC (35–40%) (7).
Although HER2-low BC accounts for a high proportion of all BC, whether HER2-low can be used as an independent subtype needs to be analyzed comprehensively in terms of clinicopathological features, prognosis, and biological characteristics.
- Clinicopathological features: it has been reported that, compared with HER2-zero tumors, HER2-low tumors had a higher proportion in HR+ tumors; in addition, grade 3 tumors have a lower proportion of HER2-low and HER2-low tumors have lower Ki67 value (8). However, it has been proposed that the differences in clinicopathological features between HER2-low and HER2-zero are driven by differences in ER expression rather than by HER2-low (9). A real-world study, including 65,035 patients with BC, suggested that HER2-low tumors were significantly associated with histologic subtype, a higher ER, and lower progesterone receptor expression in the ER+ cohort, whereas within the ER-cohort, HER2-low tumors were associated with a lower tumor grade (10). Another single center study also indicated that HER2-low/ER+ early-stage BC was associated with a lower grade and Oncotype DX recurrence score (11).
- Prognosis: multiple retrospective studies have analyzed the prognosis of HER2-low BC but with inconsistent findings. One study reported that in HER2-negative early BC (EBC) patients receiving neoadjuvant therapy, the disease-free survival (DFS) of HER2-low patients was superior to that of HER2-zero patients, mainly from patients with non-pathological complete response (non-pCR) (8). In addition, another study suggested that HER2-low expression was associated with better survival in HR+ BC patients with high Oncotype Dx RSs. Among patients who received adjuvant chemotherapy with a high Oncotype Dx RS [26–100], those with HER2-low tumors had higher survival (12). A meta-analysis of 42 studies, which included 1,797,175 patients, indicated that HER2-low status appears to be associated with a slightly increased overall survival (OS) both in the advanced [hazard ratio =0.94, 95% confidence interval (CI): 0.89–0.98, P=0.008] and early settings (hazard ratio =0.90, 95% CI: 0.85–0.95, P<0.001), regardless of HR expression. In the early setting, HER2-low tumors seem to be associated to lower pCR rates, especially if HR-positive (13). Another meta-analysis, including 636,535 patients, also suggested that HER2-low arm showed significantly improved results for DFS and OS. The hazard ratios for DFS and OS in the HR-positive group were 0.88 (95% CI: 0.83-0.94) and 0.87 (95% CI: 0.78–0.96), respectively. In the HR-negative group, the hazard ratios for DFS and OS were 0.87 (95% CI: 0.79–0.97) and 0.86 (95% CI: 0.84–0.89), respectively (14). However, lower DFS in HER2-low patients than in HER2-zero patients has also been reported in patients with node-positive BC (15). A large-sample retrospective study analyzed the data of 392,246 patients with HER2-zero BC and 743,770 patients with HER2-low BC during the period from 2010 to 2019 using the National Cancer Database (NCDB) and revealed that there were only minimal prognostic differences between HER2-low and HER2-zero BC (16). In a large international dataset of metastatic TNBC, neither in univariable nor in multivariable analysis HER2-low showed any influence on OS (17).
- Biological characteristics: intrinsic subtype analysis with PAM50 among HER2 IHC 0, 1+, 2+ BC showed that the proportions of luminal A and luminal B subtypes gradually increased and the proportion of basal-like subtypes gradually decreased with the increase of HER2 expression (7). In addition, TP53 and PIK3CA mutation rates were lower in HER2-low BC compared to HER2-zero BC (8). However, more studies have shown that HER2-low BC had no significantly different biological characteristics from those of HER2-zero BC, with manifestations similar to those of HR+ BC or TNBC. After adjustment for HR expression, the differences in molecular characteristics between HER2-low and HER2-zero BC were not significant (9).
Thus, HER2-low BC has no special clinicopathological features or prognosis, along with unstable biological characteristics. Therefore, HER2-low should not be regarded as a distinct molecular subtype but rather as a group of tumors with varying properties. The biological characteristics of HER2-low BC may or may not depend solely on HR expression.
Detection of HER2-low advanced BC
The lack of notable clinicopathological features, prognosis, and biological characteristics does not mean that HER2-low is not clinically relevant. Rather, this biomarker has played a key role in clinical decision-making since the announcement and publication of the results of the DB-04 trial. But the detection of HER2-low BC remains a challenge. In particular, it is still difficult to differentiate between HER2-low and HER2-zero tumors.
At present, an HER2-low BC is defined as a BC with HER2 IHC score of 1+, or HER2 IHC score of 2+ with a negative ISH result; however, the difficulty in distinguishing between IHC 0 and IHC 1+ poses a challenge to pathologists. Researchers from Yale University investigated the survey data from CAP including scores over 2 years from 1,391 to 1,452 laboratories of 40 ERBB2 cores from each laboratory and found that 19% of cases read by the laboratories generate results with less than or equal to 70% concordance for IHC ERBB2 score 0 versus 1+ (18). In addition, when 18 pathologists read the scanned slides from 170 BC biopsies, there was only 26% concordance between 0 and 1+ (cut point for acceptable agreement: 90% or greater) when the research objective (to assess the concordance between 0 and 1+) was not informed.
In addition to the pathologist’s scoring, factors affecting the distinction between IHC 0 and 1+ also include the following: first, the impact of IHC assays on the detection of HER2-low remains unclear; however, it has been found that different antibodies used will affect HER2-low detection, and whether there are other factors needs to be further investigated. Although both HercepTest and VENTANA 4B5 have been approved by the US Food and Drug Administration (FDA) for the detection of HER2 status, they had significant differences in the detection rate of HER2-low BC (19). There was a high concordance between results from the HercepTest and VENTANA 4B5 assays (298 vs. 303) for HER2-zero. However, among 137 patients diagnosed with HER2-low by VENTANA 4B5, only 28 cases were detected by HercepTest, and 108 cases were diagnosed as HER-zero, indicating a significantly lower detection rate of HER2-low expression using HercepTest compared to VENTANA 4B5. On 30 September 2022, the US FDA approved the VENTANA PATHWAY anti-HER2 (4B5) antibody for use as a companion diagnostic for T-Dxd to detect HER2 expression.
Second, intra-tumor heterogeneity may affect IHC 0/1+ differentiation, and therefore increasing the specimen volume may help to avoid the effect of heterogeneity. Finally, HER2-low status is prone to dynamic changes with time and treatments. A shift between HER2-zero tumors and HER2-low tumors has been discovered in 20–40% of patients with primary and recurrent metastatic lesions (20). Therefore, it has been suggested that, in HER2-negative patients, any HER2 expression that has been present throughout the course of the disease should be considered HER2-low (20).
Moreover, study using mRNA expression data suggested that the HER2-low were a mix of tumors with reference-like (70%) and abnormally elevated (30%) expression levels of ERBB2 (21). In the former cases, HER2 expression is expected to be at physiologic levels, whereas in the latter cases, HER2 expression may be increased through transcriptional mechanisms. This result reflects another reason why it is hard to distinguish HER2-low and HER2-0.
Data from the DB-04 study showed that 78% of MBC specimens locally scored as HER2-low were confirmed as HER2-low at centralized reanalysis. Despite the lack of standard clinical assays for HER2-low, the absence of guidelines for distinguishing between HER2-zero and HER2-low, and the variations in both local testing methods and key sample features, there is significant concordance between local and centralized results. The concordance of HER2-low is related to areas and the date of collection. The lowest concordance was observed in specimens collected prior to 2013, probably due to the following: first, the specimens were stored too long; and second, there were no ASCO/CAP guidelines for HER2 testing to standardize the detection and interpretation of HER2 status.
For more than two decades, HER2 has been categorized in a dichotomous manner as positive and negative. However, the availability of novel anti-HER2 therapies has redefined the classification of HER2. The results of the DB-04 trial confirmed the benefit of T-Dxd therapy for patients with HER2-low BC, and thus the HER2 classification needs to be optimized to best identify the populations that would benefit from T-Dxd. Accordingly, a 3-tier HER2 scoring system has been used: HER2+, HER2-low, and HER2-zero. The 2023 ASCO/CAP update and ESMO expert consensus statements highlight recommendations to distinguish IHC 0 from 1+ (5,22). The ongoing phase 3 randomized DESTINY-Breast06 trial will include patients with HER2-ultralow, which has an IHC between 0 and 1+, defined as the membrane staining that is faint/barely perceptible and within ≤10% of the tumor cells. This concept may further expand the population that benefits from T-Dxd. With the validation of new quantitative HER2 assays, HER2 expression profiling may be further extended to all patients with HER2-non-amplified tumors, allowing an accurate quantitative description of HER2 expression levels and informing clinical decisions.
Researchers have been contributing to explore the limitations of traditional diagnostic methods, propose advanced diagnostic approaches, and suggest novel techniques for precise measurement of HER2. qRT-PCR was employed to distinguish between HER2 IHC score 1+ and score 0 tumors (23,24). The result suggested that IHC may not accurately reflect HER2 levels in some samples, and ERBB2 mRNA expression might be more relevant to the prognosis of HER2-low cohort. In addition, Combination of quantitative immunofluorescence and mass spectrometry was found to be a more accurate measurement of HER2 protein in tissue sections (25). Furthermore, automated computation was also introduced to help determine precise cutoffs of HER2-low diagnosis (21).
The main purpose of tumor classification is to enable better treatment. HER2-low detection has been successful in identifying patients with HER2-non-amplified tumors who may benefit from T-Dxd. However, great challenges remain in interpreting this biomarker. First, the impact of the spatial and temporal evolution of HER2 expression needs to be further clarified to optimize patient selection in clinical practice. Second, we need to validate new HER2 assays to raise the consistency of HER2 scores within low expression levels and to expand the range of HER2 expression. Finally, we need to dynamically update the definition of HER2-low as our knowledge evolves. HER2-low reflects the level of HER2 expression that allows the use of anti-HER2 ADCs but is by no means the lowest level of expression. This new trend brings vast opportunities. Further research in this field will unlock the therapeutic potential of novel anti-HER2 ADCs and hopefully benefit more BC subpopulations.
Advances in the treatment of HER2-low advanced BC
Traditional monoclonal antibodies and small molecular tyrosine kinase inhibitors (TKIs) have little therapeutic effect on BC with HER2-low expression. For a long time, HER2-low BC has been included in the treatment sequence of HER2− BC, including HR+/HER2− and TNBC. T-Dxd is the third-generation ADC, which is formed by coupling trastuzumab with a highly active topoisomerase I inhibitor Dxd through a cleavable tetrapeptide linker. The drug-to-antibody ratio is as high as 8:1, and it has an anti-tumor “bystander effect”. With the improvement of the mechanism of action of these drugs, T-DXd has not only successfully replaced T-DM1 as a new standard for second-line treatment of HER2-positive advanced BC, but also demonstrated positive anti-tumor activity in HER2-low advanced BC.
DB-04 was the first phase 3 trial conducted in patients with HER2-low MBC (3). Eligible patients have previously been treated with one or two lines of chemotherapy, and patients with HR+ disease have received at least one line of endocrine therapy. The patients were randomized in a 2:1 ratio to receive either T-Dxd or treatment of physician’s choice (TPC). The primary endpoint of the study was progression-free survival (PFS) in the HR+ cohort. It was found that the T-Dxd group had a median PFS (mPFS) of 10.1 months in HR+ cohort, compared to 5.4 months in the control group (P<0.001). The PFS benefit was also observed among all patients (hazard ratio =0.50; 95% CI: 0.40–0.63; P<0.001) and the HR-negative cohort (hazard ratio =0.46; 95% CI: 0.24–0.89). In addition, T-Dxd also improved OS in HR+ cohort (hazard ratio =0.64; 95% CI: 0.48–0.86; P=0.0028) and all patients (hazard ratio =0.64; 95% CI: 0.49–0.84; P=0.0010). This survival improvement was also observed in the small number of HR− cohort, which belonged to TNBC. Compared with the TPC group, the T-Dxd group had a significantly higher objective response rate (ORR) (52.6% vs. 16.3%, respectively, in the HR+ cohort; and 50% vs. 16.7%, respectively, in the HR− cohort).
On 5 August 2022, the US FDA approved T-Dxd for the treatment of adult patients with unresectable or metastatic HER2-low BC who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy. Data presented at the San Antonio Breast Cancer Symposium (SABCS) 2022 showed similar benefits in subgroups with different disease burden, rate of progression, HER2 IHC status, prior lines of chemotherapy, age, baseline CNS metastases and prior anthracyclines treatment (26). T-Dxd was shown to achieve a mPFS of 10.0 months and an ORR of 50.6% in HR+ patients with prior CDK4/6 inhibitor (CDK4/6i) use. Data on biomarkers released at the 2023 American Society of Clinical Oncology (ASCO) annual meeting, which revealed that the ORR of T-Dxd was superior to that of TPC regardless of intrinsic subtypes (estimated by PAM50), ESR1 mutation status, PIK3CA mutation status, or CDK4/6i resistance markers (including CCND1, CCNE1, CDK6, and FGFR1/2 amplification and RB1, PTEN, RAS, AKT1, ERBB2, and FAT1 mutations) (27). In patients treated with T-Dxd, the ORR was higher in the HER2-enriched subtype than in all patients; in the TPC group, however, the ORR was lower in the HER2-enriched subtype than in all patients. In the HER2-enriched subtype T-Dxd group also achieved an obviously improved mPFS of 11.0 months compared with 2.7 months in TPC group (hazard ratio =0.15; 95% CI: 0.05–0.40). In patients who had not received prior CDK4/6i therapy and were negative for CDK4/6i resistance markers, mPFS reached 17.9 months. According to the data on patients with low ER expression (ER IHC 1–10%) released at the ESMO Breast Cancer Congress 2023, the benefit of T-Dxd treatment was independent of ER status, and HER2-low patients with low ER expression could also benefit from this therapy (28).
There were two cohorts in the DB-04 trial: HER2-low HR+ and HER2-low HR−, which belong to HR+/HER2− and TNBC, respectively, in the original diagnostic pathway. CDK4/6i have secured a role in the treatment of HR+/HER2− MBC in the first-line setting; however, the treatment strategies after CDK4/6i treatment remain controversial. On the one hand, chemotherapy may be considered, as a real-world study has shown an mPFS of 7.2 months for switching to chemotherapy after first-line CDK4/6i treatment for advanced tumors (29). On the other hand, the endocrine therapy may be continued. Endocrine therapy options include single-agent endocrine therapy. In the ELAINE 1 trial, single-agent treatment with lasofoxifene resulted in an mPFS of 6.04 months (30); in contrast, the mPFS of single-agent elacestrant treatment was only 2.79 months in the EMERALD study (31). Thereupon, endocrine therapy plus targeted therapy may be alternatively considered. In the BYLieve cohort study in which patients with PIK3CA mutations were included, after progression with previously treated with CDK4/6i, the targeted therapy was switched with alpelisib, and the endocrine therapy was switched to another. The mPFS was 7.3 months for those who received alpelisib + fulvestrant (cohort A) and 5.7 months for those who received alpelisib + letrozole (cohort B) (32). The rechallenging CDK4/6i post progression was explored in the MAITAIN study, with an mPFS of 5.29 months in the group treated with ribociclib + endocrine therapy (33). Multiple targeted therapy combinations were explored in the TRINITI-1 study, among which ribociclib + everolimus + exemestane yielded an mPFS of 5.7 months (34). Alternatively, ADC therapy can also be considered. There have been two well-known studies using ADCs after CDK4/6i progression. The TROPiCS-02 study reported an mPFS of 5.5 months with sacituzumab govitecan treatment (35), and in this study patients with at least one line endocrine therapy, at least two but no more than four lines of chemotherapy were included. In the DB-04 study (3), T-Dxd achieved an mPFS of 10.1 months. Therefore, the 2023 ESMO metastatic BC (MBC) living guidelines recommended the use of T-DXd after progression on first-line endocrine therapy plus CDK4/6i or for high visceral disease patients after progression on first line of chemotherapy (36).
In another cohort (i.e., patients originally classified as TNBC) of DB-04, chemotherapy therapy was the standard first-line treatment for patients with programmed cell death ligand 1 (PD-L1)-negative, gBRCA-wild type advanced TNBC after screening for PD-L1 status and gBRCA mutations. Until recently, single-agent chemotherapy was the standard of care treatment option for previously treated mTNBC but it is associated with short progression-free survival (PFS), low response rates, and significant toxicity (37,38). In the second-line (2L) or later mTNBC setting, single-agent chemotherapy results in a mPFS of <3 months and an ORR of 11% (38-41). In the ASCENT study, the advanced TNBC patients, who have received more than two previous lines of chemotherapy, had an mPFS of 5.7 months after sacituzumab govitecan treatment (42). The phase III ASCENT trial led to the approval of sacituzumab govitecan (SG) (10 mg/kg, d1, 8 q3w) in patients with advanced or metastatic TNBC who have received ≥2 prior systemic therapies, including ≥1 for metastatic disease. In the DB-04 study, T-Dxd achieved an mPFS of 8.5 months (3). Therefore, the 2023 ESMO MBC living guidelines recommended the use of T-Dxd for patients with advanced TNBC after failure of second-line therapy (36).
As mentioned above, there are several treatment options including endocrine therapy and chemotherapy available in HR+ BC, immunotherapy and chemotherapy in TNBC, and the ADCs in both HR+ and TNBC. Researcher and clinicians are devoted to proposing the optimal sequencing of these agents for maximum clinical benefit while maintaining the quality of life (43). With current evidence, a sequencing strategy for HR+ and HR− HER2-low MBC is recommended (Figure 1).
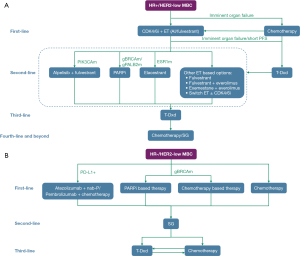
Although the results of the DB-04 study confirmed the therapeutic efficacy of T-Dxd in HER2-low BC, there is a lack of evidence from large-scale studies on the efficacies of various ADCs in HER2-low BC. Vidiximab (RC48) is an ADC independently developed in China. Its structure includes antibodies against the extracellular domain (ECD) of HER2, linker, and cytotoxic monomethylauristatin E (MMAE). The C003 CANCER study enrolled a subset of patients with HER2-low advanced BC who had received ≥3 lines of therapies, among whom the ORR was 39.6% and mPFS was 5.7 months (44). SHR-A1811 is an ADC comprised of a humanized anti-HER2 monoclonal antibody (trastuzumab), a cleavable linker, and a DNA topoisomerase I inhibitor payload. Phase I clinical study data of SHR-A1811 was published on AACR in 2023. This study recruited 77 patients with BC with HER2-low BC who received at least 3 lines treatment in metastatic setting. The confirmed ORR was 49.4% (38/77) (95% CI: 37.8–61.0%), and the 6-month PFS rate was 63.8% (95% CI: 47.8–76.0%) (45). This study preliminarily verified that SHR-A1811 was well-tolerated and showed promising antitumor activity in heavily pretreated HER2-low BC. Novel ADCs are currently being all-around tested for the treatment of HER2-low BC patients, with the aim of bringing more treatment strategies to this patient subgroup.
While these treatments can be effective, they may also be associated with side effects. The common ones are nausea and lowered blood counts, and these can be managed with medication. However, a small number of people who receive T-Dxd have interstitial lung disease (ILD). This risk has been noted from the very first studies of the drug. In the DESTINY-Breast04 trial, it occurred in about 12% of patients who received T-DXd (3). Based on available reports, a multidisciplinary guideline has been produced on proactive monitoring, diagnosis, and management of T-Dxd related ILD. However, there are still many areas waiting for future investigation (46).
Conclusions
The existing evidence fails to substantiate HER2-low BC as a novel subtype. Nevertheless, the characterization of HER2-low has facilitated the identification of patients with HER2-negative tumors who can potentially benefit from T-DXd, thereby underscoring the considerable importance of HER2-low. Previous attempts have been undertaken to identify HER2-positive tumors. The limitations of current HER2 testing assays may impede the detection of HER2-low BC. Consequently, enhancing the detection of HER2-low tumors presents a significant challenge. The data derived from various clinical trials indicates a significant survival advantage associated with T-Dxd. We are truly inspired to witness the great revolution with the emergence of T-Dxd. As Dr. Shanu Modi, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York, New York, USA, commented at ESMO Congress 2021, “The data derived from various clinical trials indicates a …statistically… significant survival advantage associated with T-Dxd.”
Acknowledgments
Funding: This work was supported by Wu Jieping Medical Foundation Clinical Research Project (320.6750.2021-10-23).
Footnote
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-48/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-48/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Pondé N, Brandão M, El-Hachem G, et al. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev 2018;67:10-20. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36:2105-22. [Crossref] [PubMed]
- Tarantino P, Viale G, Press MF, et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann Oncol 2023;34:645-59. [Crossref] [PubMed]
- Tarantino P, Hamilton E, Tolaney SM, et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J Clin Oncol 2020;38:1951-62. [Crossref] [PubMed]
- Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021;7:1. [Crossref] [PubMed]
- Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 2021;22:1151-61. [Crossref] [PubMed]
- Tarantino P, Jin Q, Tayob N, et al. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol 2022;8:1177-83. [Crossref] [PubMed]
- Baez-Navarro X, van Bockstal MR, Andrinopoulou ER, et al. HER2-Low Breast Cancer: Incidence, Clinicopathologic Features, and Survival Outcomes From Real-World Data of a Large Nationwide Cohort. Mod Pathol 2023;36:100087. [Crossref] [PubMed]
- Hu Y, Jones D, Zhao W, et al. Incidence, Clinicopathologic Features, HER2 Fluorescence In Situ Hybridization Profile, and Oncotype DX Results of Human Epidermal Growth Factor Receptor 2-Low Breast Cancers: Experience From a Single Academic Center. Mod Pathol 2023;36:100164. [Crossref] [PubMed]
- Roy AM, Jiang C, Perimbeti S, et al. Oncotype Dx Score, HER2 Low Expression, and Clinical Outcomes in Early-Stage Breast Cancer: A National Cancer Database Analysis. Cancers (Basel) 2023;15:4264. [Crossref] [PubMed]
- Molinelli C, Jacobs F, Agostinetto E, et al. Prognostic value of HER2-low status in breast cancer: a systematic review and meta-analysis. ESMO Open 2023;8:101592. [Crossref] [PubMed]
- Ergun Y, Ucar G, Akagunduz B. Comparison of HER2-zero and HER2-low in terms of clinicopathological factors and survival in early-stage breast cancer: A systematic review and meta-analysis. Cancer Treat Rev 2023;115:102538. [Crossref] [PubMed]
- Gilcrease MZ, Woodward WA, Nicolas MM, et al. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol 2009;33:759-67. [Crossref] [PubMed]
- Peiffer DS, Zhao F, Chen N, et al. Clinicopathologic Characteristics and Prognosis of ERBB2-Low Breast Cancer Among Patients in the National Cancer Database. JAMA Oncol 2023;9:500-10. [Crossref] [PubMed]
- Gampenrieder SP, Dezentjé V, Lambertini M, et al. Influence of HER2 expression on prognosis in metastatic triple-negative breast cancer-results from an international, multicenter analysis coordinated by the AGMT Study Group. ESMO Open 2023;8:100747. [Crossref] [PubMed]
- Fernandez AI, Liu M, Bellizzi A, et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol 2022;8:1-4. [Crossref] [PubMed]
- Scott M, Vandenberghe ME, Scorer P, et al. Prevalence of HER2 low in breast cancer subtypes using the VENTANA anti-HER2/neu (4B5) assay. J Clin Oncol 2021;39:1021. [Crossref]
- Tarantino P, Gandini S, Nicolò E, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer 2022;163:35-43. [Crossref] [PubMed]
- Guvakova MA. Automated Classification of Breast Cancer Across the Spectrum of ERBB2 Expression Focusing on Heterogeneous Tumors With Low Human Epidermal Growth Factor Receptor 2 Expression. JCO Clin Cancer Inform 2023;7:e2300013. [Crossref] [PubMed]
- Wolff AC, Somerfield MR, Dowsett M, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol 2023;41:3867-72. [Crossref] [PubMed]
- Atallah NM, Toss MS, Green AR, et al. Refining the definition of HER2-low class in invasive breast cancer. Histopathology 2022;81:770-85. [Crossref] [PubMed]
- Liu Y, Lv H, Shen M, et al. ERBB2 mRNA expression to distinguish HER2-low/neg breast cancer prognosis. J Clin Oncol 2023;41:569. [Crossref]
- Moutafi M, Robbins CJ, Yaghoobi V, et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest 2022;102:1101-8. [Crossref] [PubMed]
- Harbeck N, Modi S, Jacot W, et al. Abstract P1-11-01: Trastuzumab deruxtecan vs treatment of physician’s choice in patients with HER2-low unresectable and/or metastatic breast cancer: Subgroup analyses from DESTINY-Breast04. Cancer Res 2023;83:P1-11-01.
- Modi S, Niikura N, Yamashita T, et al. Trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low, hormone receptor-positive (HR+) unresectable and/or metastatic breast cancer (mBC): Exploratory biomarker analysis of DESTINY-Breast04. J Clin Oncol 2023;41:1020. [Crossref]
- Cameron DA, Jacot W, Yamashita T, et al. 192MO - DESTINY-Breast04 subgroup analyses of trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with human epidermal growth factor 2 (HER2)-low, estrogen-receptor (ER) expression immunohistochemistry (IHC) 0-10% metastatic breast cancer (mBC). Ann Oncol 2023;8:101223.
- Li Y, Li W, Gong C, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol 2021;13:17588359211022890. [Crossref] [PubMed]
- Goetz MP, Plourde P, Stover DG, et al. Open-label, randomized study of lasofoxifene (LAS) vs fulvestrant (Fulv) for women with locally advanced/metastatic ER+/HER2- breast cancer (mBC), an estrogen receptor 1 (ESR1) mutation, and disease progression on aromatase (AI) and cyclin-dependent kinase 4/6 (CDK4/6i) inhibitors. Ann Oncol 2022;33:S1387-8. [Crossref]
- Bardia A, Aftimos P, Bihani T, et al. EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol 2019;15:3209-18. [Crossref] [PubMed]
- Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol 2021;22:489-98. [Crossref] [PubMed]
- Kalinsky K, Accordino MK, Chiuzan C, et al. Randomized Phase II Trial of Endocrine Therapy With or Without Ribociclib After Progression on Cyclin-Dependent Kinase 4/6 Inhibition in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: MAINTAIN Trial. J Clin Oncol 2023;41:4004-13. [Crossref] [PubMed]
- Bardia A, Hurvitz SA, DeMichele A, et al. Phase I/II Trial of Exemestane, Ribociclib, and Everolimus in Women with HR(+)/HER2(-) Advanced Breast Cancer after Progression on CDK4/6 Inhibitors (TRINITI-1). Clin Cancer Res 2021;27:4177-85. [Crossref] [PubMed]
- Rugo HS, Bardia A, Marmé F, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol 2022;40:3365-76. [Crossref] [PubMed]
- Curigliano G, Castelo-Branco L, Gennari A, et al. ESMO Metastatic Breast Cancer Living Guideline v1.1 May 2023. Available online: https://www.esmo.org/living-guidelines/esmometastatic-breast-cancer-living-guideline
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623-49. [Crossref] [PubMed]
- Li CH, Karantza V, Aktan G, et al. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res 2019;21:143. [Crossref] [PubMed]
- Park IH, Im SA, Jung KH, et al. Randomized Open Label Phase III Trial of Irinotecan Plus Capecitabine versus Capecitabine Monotherapy in Patients with Metastatic Breast Cancer Previously Treated with Anthracycline and Taxane: PROCEED Trial (KCSG BR 11-01). Cancer Res Treat 2019;51:43-52. [Crossref] [PubMed]
- Perez EA, Patel T, Moreno-Aspitia A. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res Treat 2010;121:261-71. [Crossref] [PubMed]
- Pivot X, Marmé F, Koenigsberg R, et al. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol 2016;27:1525-31. [Crossref] [PubMed]
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 2021;384:1529-41. [Crossref] [PubMed]
- Roy AM, Kumarasamy VM, Dhakal A, et al. A review of treatment options in HER2-low breast cancer and proposed treatment sequencing algorithm. Cancer 2023;129:2773-88. [Crossref] [PubMed]
- Wang J, Liu Y, Zhang Q, et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J Clin Oncol 2021;39:1022. [Crossref]
- Yao H, Yan M, Tong Z, et al. Abstract CT175: Safety, tolerability, pharmacokinetics, and antitumor activity of SHR-A1811 in HER2-expressing/mutated advanced solid tumors: A global phase 1, multi-center, first-in-human study. Cancer Res 2023;83:CT175. [Crossref]
- Swain SM, Nishino M, Lancaster LH, et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev 2022;106:102378. [Crossref] [PubMed]
Cite this article as: Wu Q, Xu L. Challenges in HER2-low breast cancer identification, detection, and treatment. Transl Breast Cancer Res 2024;5:3.


