
A case report of low-dose apatinib in the treatment of advanced triple-negative breast cancer
Highlight box
Key findings
• Low-dose apatinib has achieved good results in a patient with metastatic triple-negative breast cancer who failed chemotherapy, and the treatment is well tolerated.
What is known and what is new?
• Recent studies have shown that anti-angiogenic drugs play an important role in advanced triple-negative breast cancer. Some small molecule tyrosine kinase inhibitors with anti-angiogenic effects, such as apatinib, have also shown partial efficacy in clinical studies. However, in clinical practice, the efficacy of using it alone is limited.
• The low dose of apatinib in this case achieved a progression free survival period of 45 months, with good long-term oral tolerance, and is relatively rare in triple negative cancers with limited treatment options.
What is the implication, and what should change now?
• The good management of this case takes into account the long-term anti-tumor efficacy, safety, and tolerance, providing a reference for future treatment choices for triple negative metastatic cancer.
Introduction
Triple negative breast cancer (TNBC) is a special type of breast cancer, which lacks the expression of estrogen receptor (ER), progesterone receptor and human epidermal growth factor receptor 2 (HER2) genes. The incidence rate of TNBC accounts for 10–17% in breast invasive ductal carcinoma. The treatment of TNBC is particularly challenging due to the lack of recognized molecular biological targets. Unfortunately, TNBC is highly proliferative and invasive, the disease progresses rapidly, the risk of recurrence is high, and the prognosis is poor (1). Chemotherapy is the main treatment of TNBC, and new drugs and strategies are urgently needed. At present, many studies have shown that angiogenesis inhibitors play an important role in advanced TNBC. There are also some small molecule tyrosine kinase (TK) inhibitors with anti-angiogenic effects, such as apatinib, which have also shown some efficacy in clinical studies.
Angiogenesis is one of the signs of cancer, which is crucial for tumor growth, development and metastasis. Anti-angiogenesis is an important anti-cancer strategy (2). Vascular endothelial growth factor (VEGF) signal plays an important role in angiogenesis by activating VEGF receptor (VEGFR) (2). The VEGFR family involves three molecular subtypes (VEGFR-1, VEGFR-2 and VEGFR-3), which are type II transmembrane proteins characterized by TK activity (3). Among them, VEGFR-2 is mainly related to the pathological excessive formation of blood vessels in a variety of solid tumors (4).
Apatinib is a new generation of small molecule TK inhibitors, which can highly selectively inhibit the phosphorylation of VEGFR-2, mainly through the anti-angiogenesis pathway, and inhibit tumor proliferation and metastasis. Recently, apatinib has shown satisfactory efficacy in many cancers, such as gastric cancer (5), breast cancer (6), and nasopharyngeal cancer (7). At the same time, it has acceptable toxicity. It also shows that the bioavailability in the human body is relatively high, and it is convenient to take orally in clinical practice, with a certain degree of safety, which is easily tolerated by patients (8). Here, we report a typical case of TNBC with lung metastasis. After chemotherapy failure, oral apatinib reached clinical complete response (CR), and reached 45 months of progression-free survival. This case provides a reference for the treatment of triple-negative metastatic breast cancer in the future. We present this case in accordance with the CARE reporting checklist (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-24/rc).
Case presentation
The patient was diagnosed with TNBC at the age of 55 in April 2015. After right breast-conserving surgery, the pathological stage was T1N0M0. After the operation, patients received AC regimen (pirarubicin hydrochloride 100 mg, 50 mg/m2, dl and cyclophosphamide 1,000 mg, 60 mg/m2, 21 days as a cycle) for 4 cycles, followed by T regimen [paclitaxel 300 mg (175 mg/m2, 14 days as a cycle) for 4 cycles]. After that, she received adjuvant radiotherapy, completed 6MV-X Dt 5,000 cGy/25 f, and locally added to Dt 6,000 cGy/30 f. Cellular immunotherapy was performed from October 2015 to January 2016. On March 22, 2017, when right lower lung metastasis was found, NX regimen (vinorelbine capsule 100 mg, 56 mg/m2, dl, 8 and capecitabine 3,500 mg, 1,989 mg/m2, days 1–14, 21 days as a cycle) was selected as the first-line chemotherapy for 8 cycles, with the best effect reaching disease stabilization (SD). The main adverse reactions were anorexia, liver function damage, and weight loss. On October 19, 2017, there was progress in the disease, which showed that the lung metastasis was enlarged. The patient refused chemotherapy. On October 21, 2017, the patient began to take 500 mg of apatinib mesylate orally for treatment. Grade 1 hand foot syndrome, grade 2 proteinuria, grade 3 diarrhea, and grade 3 hypertension occurred. After 5 months, 500 mg of apatinib mesylate was taken for 5 days and 2 days off, but still had grade 2 diarrhea and grade 1 proteinuria, the blood pressure was well controlled. After 2 months, it was reduced to 500 mg of apatinib mesylate for 4 days and 3 days off. In September 2019, the pulmonary lesions were close to CR (4 mm). According to RECIST 1.1 (9), the treatment evaluation result is a partial response (PR; Figure 1). On September 9, 2019, the patient reduced the dosage of the drug to 500 mg of apatinib mesylate for 3 days and 4 days off. During this period, the adverse reactions of the patients were mild (grade 1 hypertension, grade 1 proteinuria, grade 1 diarrhea, grade 1 hand-foot syndrome, grade 1 hypothyroidism), with good tolerance (Table 1). By June 2021, a reexamination of positron emission tomography (PET) computed tomography (CT) showed that lymph nodes in the neck, mediastinum, and right hilar lung were metastatic, and left adrenal gland mass was considered to be metastatic. Metastatic tumor of the left frontal lobe. The progression-free survival was 45 months (Figure 2). This study was conducted in accordance with the Helsinki Declaration (revised in 2013). This study has been approved by the Ethics Committee of General Hospital of Ningxia Medical University (Ethics Committee registration number 2023-28) and obtained informed consent from the patient.
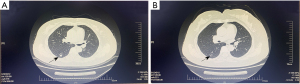
Table 1
| Course of treatment | Treatment process | |||
|---|---|---|---|---|
| 2017.10–2018.03 | 2018.03–2018.05 | 2018.05–2019.09 | 2019.09–2021.06 | |
| Dose (apatinib) | 500 mg q.d. | 500 mg for 5 days and 2 days off | 500 mg for 4 days and 3 days off | 500 mg for 3 days and 4 days off |
| Efficacy evaluation | PR | PR | PR | PR |
| Adverse reactions | Grade 1 hand-foot syndrome, grade 2 proteinuria, grade 3 diarrhea and grade 3 hypertension | Grade 2 diarrhea and grade 1 proteinuria, the blood pressure was well controlled | Grade 1 hypertension, grade 1 proteinuria, grade 1 diarrhea, grade 1 hand foot syndrome, grade 1 hypothyroidism | |
PR, partial response.

Discussion
TNBC accounts for 10–15% of all breast cancer cases. TNBC is a particularly aggressive and heterogeneous breast cancer. Compared with other breast cancer subtypes, because there is no specific therapeutic target, the prognosis is usually poor. At present, chemotherapy is still the standard treatment method for TNBC. In recent years, some progress has been made in the application of new drugs for specific subtypes of programmed death ligand 1 (PD-L1) + tumor or embryonic BRCA mutation. However, only a small part of these patients have responded to immune checkpoints or Poly (ADP-ribose) polymerase (PARP) inhibitors, and even patients who have responded often have drug resistance and relapse, so the treatment of TNBC remains a clinical challenge, especially, patients who fail in first-line or second-line treatment, they urgently need new drugs and treatment strategies.
Angiogenesis is one of the signs of cancer, which is crucial for tumor growth, development and metastasis. Anti-angiogenesis is an important anti-cancer strategy. VEGF signal plays an important role in angiogenesis by activating VEGFR. The VEGFR family involves three molecular subtypes (VEGFR-1, VEGFR-2 and VEGFR-3), which are type II transmembrane proteins characterized by TK activity. Among them, VEGFR-2 is mainly related to the pathological excessive formation of blood vessels in various solid tumors.
Apatinib is a drug independently researched and developed in China. It is a new generation of small molecule TK inhibitors. It has potential anti-angiogenesis and anti-tumor functions. It can selectively bind and inhibit VEGFR-2, inhibition of VEGF-stimulated endothelial cell migration and proliferation, and reduce tumor microvessel density, thereby inhibiting tumor proliferation and metastasis. In addition, apatinib can inhibit a variety of TKs related to tumor genesis and progression, thereby inhibiting tumor angiogenesis, in order to achieve the purpose of anti-tumor (10,11).
Apatinib is the first small molecule anti-angiogenesis targeted drug that has been proved to be safe and effective for advanced gastric cancer that has failed standard chemotherapy in the world. In October 2014, it was approved by the State Food and Drug Administration to be marketed for the third-line or above treatment of advanced gastric adenocarcinoma or gastric-esophageal junction adenocarcinoma. At present, although apatinib has not been approved for the treatment of breast cancer, clinical trials for breast cancer have been carried out. In recent years, a series of studies have shown that apatinib has anti-tumor activity in several other solid tumors, including non-small cell lung cancer (12) and breast cancer (6), and has good tolerance in previous studies (13,14). In a multicenter phase II study, the efficacy and safety of apatinib in patients with metastatic TNBC after multi-line therapy were also evaluated (6). The long-term benefit of single-agent apatinib is rarer. The patients reported in this article chose single drug apatinib after failure of first-line chemotherapy, and achieved clinical CR after 2 years of oral apatinib treatment. The overall progression free survival time reached 45 months, which is a very rare clinical success case.
The adverse reactions of long-term oral of apatinib are also the focus of attention. The standard dose of 750 or 500 mg is once a day, and the common adverse reactions such as proteinuria, hypertension and hand-foot syndrome occur frequently. In clinical practice, patients often feel relieved after taking medicine, but have to stop treatment due to uncontrollable hypertension and proteinuria, which affects the overall effect of treatment. A phase IIb clinical study found that the reduction of drug concentration can reduce the occurrence and degree of adverse reactions, and the efficacy is not affected (6). The patient took 500 mg q.d. orally at the time of initial diagnosis, and the main adverse reactions were: elevated blood pressure, hand-foot syndrome, hypothyroidism, proteinuria, diarrhea. Especially diarrhea and blood pressure rise to grade 3. After 5 months, the condition was evaluated to be stable. Because of adverse reactions, after 2 months, the drug dose was adjusted to 500 mg for 4 days and 3 days off. The clinical CR was reached in the reexamination in September 2019, and the dosage was adjusted to 500 mg for 3 days and 4 days off. The adverse reactions were all grade 1. The overall progression-free survival time reached 45 months, with mild adverse reactions. It can be seen that the reduction of the drug concentration of apatinib can reduce the occurrence and degree of adverse reactions, and the efficacy is not affected. It is consistent with the clinical research results. At the same time, low-dose apatinib not only alleviates the adverse reactions and economic burden of patients, but also achieves better efficacy, and obtains a longer disease progression-free survival period, which provides a new strategy for the treatment of advanced breast cancer.
The patient was adjusted to albumin paclitaxel monotherapy after the disease progressed again, and the gene detection was improved. The results are shown in Figure 3. KEYNOTE-355 research results show that for advanced triple-negative breast cancer with high PD-L1 expression, pembrolizumab combined with chemotherapy can significantly improve progression-free survival compared with chemotherapy alone. The detection result of this patient shows that PD-L1 is highly expressed. If the efficacy of albumin paclitaxel was poor, chemotherapy combined with PD-1treatment could be considered.

Apatinib mesylate monotherapy for multidrug-resistant advanced breast cancer has a significant short-term effect, and has good drug resistance. The adverse reactions are less and can be controlled, thus improving the quality of life of patients. It has become an effective treatment for multidrug resistant breast cancer. This case proves that apatinib mesylate monotherapy has an ideal effect in the treatment of multidrug-resistant advanced breast cancer. Low-dose medication can reduce the adverse reactions of drugs and the economic burden of patients, achieve the same treatment effect, and have better tolerance. It is worthy of clinical promotion and application.
Conclusions
Here, we report a case of advanced metastatic TNBC. After the first-line chemotherapy progress, she was adjusted to apatinib mesylate, and serious adverse reactions occurred during the treatment. By adjusting the drug dosage and low-dose apatinib treatment, the efficacy is close to clinical CR, achieving a progression-free survival of 45 months.
Through the treatment of this patient, we found that low-dose apatinib may be a promising anti-tumor drug for treating triple-negative cancer patients, requiring more samples for validation. This case can provide a reference for the treatment selection of triple negative metastasis of cancer in the future.
Acknowledgments
Funding: This study was conducted by the Ningxia Natural Science Foundation, “Research on the Role of the Epigenetic Factor YY1 in Regulating breast cancer-derived Exosomes to Affect EMT” (No. 2023AAC03633), “TIM33 in Exosomes by Promoting TGF-β Research on the role of the signal network in inhibiting epithelial-mesenchymal transformation of triple-negative breast cancer” (No. 2020AAC03384).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-24/rc
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-24/prf
Conflicts of Interest: All authors have completed the ICMJE unified disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-24/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Helsinki Declaration (revised in 2013). This study has been approved by the Ethics Committee of General Hospital of Ningxia Medical University (Ethics Committee registration number 2023-28) and obtained informed consent from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009;9:29-33. [Crossref] [PubMed]
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011-27. [Crossref] [PubMed]
- Young HS, Summers AM, Bhushan M, et al. Single-nucleotide polymorphisms of vascular endothelial growth factor in psoriasis of early onset. J Invest Dermatol 2004;122:209-15. [Crossref] [PubMed]
- Roviello G, Ravelli A, Polom K, et al. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187-91. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]
- Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961-9. [Crossref] [PubMed]
- Peng QX, Han YW, Zhang YL, et al. Apatinib inhibits VEGFR-2 and angiogenesis in an in vivo murine model of nasopharyngeal carcinoma. Oncotarget 2017;8:52813-22. [Crossref] [PubMed]
- Zhang J, Jiang H, Zhu M, et al. Analysis of therapeutic effect and predictive factors of apatinib in the treatment of advanced lung cancer. The Journal of Practical Medicine 2017;33:3845-6.
- European Organisation For Research And Treatment Of Cancer. RECIST Working Group. RECIST 1.1.
- Rahimi N. Vascular endothelial growth factor receptors: molecular mechanisms of activation and therapeutic potentials. Exp Eye Res 2006;83:1005-16. [Crossref] [PubMed]
- Ding J, Chen X, Dai X, et al. Simultaneous determination of apatinib and its four major metabolites in human plasma using liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 2012;895-896:108-15. [Crossref] [PubMed]
- Wu D, Liang L, Nie L, et al. Efficacy, safety and predictive indicators of apatinib after multilines treatment in advanced nonsquamous nonsmall cell lung cancer: Apatinib treatment in nonsquamous NSCLC. Asia Pac J Clin Oncol 2018;14:446-52. [Crossref] [PubMed]
- Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [Crossref] [PubMed]
- Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [Crossref] [PubMed]
Cite this article as: Lv Y, Zhang H, Zhao Y, Zhang H, Wang T. A case report of low-dose apatinib in the treatment of advanced triple-negative breast cancer. Transl Breast Cancer Res 2024;5:8.


