
Evolution of localization methods for non-palpable breast lesions: a literature review from a translational medicine perspective
Introduction
Background
Breast cancer remains one of the most common malignancies worldwide, contributing 12.5% of the total number of new cases diagnosed in 2020, the latest year available, according to World Cancer Research Fund (1). With improved screening programs, an increasing number of breast cancers are being detected at earlier stages, often presenting as non-palpable lesions on imaging studies (2). These non-palpable breast lesions, whether benign or malignant, pose a unique challenge to the surgical management of breast disease.
Rationale and knowledge gap
Accurate localization of non-palpable breast lesions is crucial for successful breast-conserving surgery (BCS). This procedure aims to remove the tumor with an adequate margin of normal tissue while preserving as much of the breast as possible (3). The localization step ensures that the surgeon can accurately identify and excise the lesion, aiming to achieve clear margins and improve cosmetic outcomes while minimizing the volume of healthy tissue removed. The evolution of localization methods for non-palpable breast lesions has been driven by the need to overcome the limitations of traditional techniques, improve surgical outcomes, and enhance patient experience. This evolution has been characterized by a shift from invasive and uncomfortable procedures to less invasive and more patient-friendly techniques.
Objective
This review will provide an in-depth analysis of the various localization methods, focusing on their translational journey from concept to clinical practice. We present this article in accordance with the Narrative Review reporting checklist (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-49/rc).
Methods
PubMed, Embase, and Scopus were searched for studies on the different localization methods for non-palpable breast lesions until September 2023 (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 3rd September 2023 |
| Databases and other sources searched | PubMed, Embase, Scopus |
| Search terms used | Non-palpable breast lesion; wire-guided localization (WGL); intraoperative ultrasound localization; breast biopsy marker; HydroMARK; radioguided occult lesion localization (ROLL); radioactive seed localization (RSL); magnetic seed localization (MSL); Magseed; radar localization; savi scout; radiofrequency identification (RFID); LOCalizer |
| Timeframe | Up until September 2023 |
| Inclusion criteria | English; any study type |
| Selection process | Literature review were conducted by the authors |
Localization methods for non-palpable breast lesions
Multiple methods have been developed throughout the past few decades to provide accurate localization of non-palpable breast lesions, and each method has been developed based on the technology and engineering available. Comparison of the different localization methods was made with respect to positive margins, re-operation rate, cost per dive and patient satisfaction (Table 2).
Table 2
| Positive margins | Re-operation rate | Cost per device | Patient satisfaction | Type of study | |
|---|---|---|---|---|---|
| Wire-guided localization | 15–22.9% (4,5) | 14.9–20.8% (4,5) | 20 USD (6) | 77.1% (4) | Literature review with pooled analysis (5), meta-nalysis of RCTs (4) |
| Intraoperative ultrasound | 5–5.4% (4,7) | 4.8–7% (4,7) | – | – | Meta-analyses of RCTs available (7), meta-analysis of RCTs (4) |
| Breast tissue marker | – | – | – | – | – |
| Radioguided occult lesion localization | 17.0–17.2% (4,5) | 9.8–12.6% (4,5) | – | 85.6% (4) | Literature review with pooled analysis (5), meta-analysis of RCTs (4) |
| Radioactive seed localization | 11.7–12.36% (4,5) | 6.8–10.3% (4,5) | 20–50 USD (6) | 80% (4) | Literature review with pooled analysis (5), meta-analysis of RCTs (4) |
| Magseed® | 13.3–20% (5,8,9) | 11.25–13.44% (5,8,9) | 400 USD (6) | 100% (4) | Literature review with pooled analysis (5), cohort study (9), systematic review and pooled analysis (8) |
| Savi Scout® | 5.6–10.6% (5) | 5.3–8.6% (5) | 450 USD (6) | – | Literature review with pooled analysis (5) |
| LOCalizer™ | – | 13.9% (10) | 550 USD (6) | – | Systematic review (10) |
RCT, randomized controlled trial.
Overview of imaging modalities used
The choice of imaging modality to guide the wire/marker placement is decided based on obtaining the best view of the target lesion whilst optimizing patient comfort (11). Ultrasound (US) is usually chosen due to real-time imaging, better patient comfort, and shorter procedure time. US-based techniques such as Doppler imaging, high frequency transducers, elastography and contrast-enhanced US are also implemented in order to obtain a more accurate characterization of breast lesions (12). Mammogram-guided localization is often the second-choice imaging, indicated in breast lesions that cannot be detected on US or in lesions with calcifications, such as extensive ductal carcinoma in-situ (DCIS). However, stereotactic localization has disadvantages, including longer procedure time, reduced patient comfort due to breast compression, and limited choice of direction of needle insertion for marker placement. Certain anatomic locations can be difficult to access mammographically, for example, lesions in extremely posterior, inferior, medial, and central positions (6). It could be challenging to adjust the depth of needle in z-axis. Also, there can be possible migration of markers when releasing the breast from compression after stereotactic guidance, this is known as the accordion effect (13).
Magnetic resonance imaging (MRI) guidance is indicated when mammography (MMG) and US fail to adequately represent the extent of the malignancy or when the findings are only observed on MRI. However, like mammogram-guided localizations, specific lesion locations can be challenging to access for localization under MRI guidance. This highlights the importance of patient positioning. One common disadvantage of magnetic seeds, radar reflectors, and radiofrequency identification tags are the unavailability of MRI-compatible delivery systems (14). Computed tomography (CT) guided localization is used in selected cases when MRI guidance is not feasible (15).
Lastly, intraoperative imaging can be performed (including specimen MMG, specimen US) after excision of breast lesion in order to ensure a complete excision, to document the removal of wire or marker, and to assess margin status, which can allow immediate re-excision if needed to (16).
Wire-guided localization (WGL)
The initial localization of non-palpable breast lesions, documented in 1966, entailed inserting a bent wire through a needle into a breast lesion under fluoroscopy guidance (17). Subsequently, in 1976, the method evolved to combine a hook wire and a needle delivery system (18). The WGL technique was initially developed in a laboratory setting, using phantoms to mimic breast tissue (19). Over the years, the technique has been refined and improved, with advancements in imaging technology greatly enhancing the accuracy of wire placement.
WGL has proven to be an effective method for breast tumor localization and remains a gold standard localization technique (20-23). The procedure involves the insertion of a wire into the breast under imaging guidance on the day of surgery. The wire acts as a roadmap for the surgeon, leading to the target lesion.
Advantages of WGL
The major advantages of WGL lie in its simple tool requirement and low cost (7). Moreover, wires can be inserted into the breast using mammographic, US, or MRI guidance (11,24,25). Not unique to WGL, MMG-guided localizations are commonly more time-consuming and less tolerable due to the breast compression and procedure length (6). Additional views and tools such as a prone table may be required for optimal visualization. It is not uncommon for malignancy, in particular DCIS to present as extensive microcalcification that may not be easy for surgeons to orientate for an adequate excision due to the difficulty in comprehending the lesion into three dimensions from two-dimensional MMGs (19). On the bright side, it is relatively straightforward to use WGL to localization via bracketing, a technique developed mainly for MMG localization to mark the outer edge of a lesion to facilitate a complete excision (26). Besides, there is no limitation between the distance of multiple wires for the ipsilateral breast.
From the historical point of view, the development and adoption of WGL marked a significant advancement in the surgical management of non-palpable breast lesions with greater precision, contributing to improved oncological and cosmetic outcomes.
Limitations of WGL
Despite its widespread use, WGL also has its drawbacks. For radiological-related limitations, WGL is highly dependent on the experience of the radiologist. For wire-related disadvantages, the risk of wire migration, either spontaneously or due to patient activity, can lead to inaccurate localization and, potentially, the wire’s complete loss (18,19).
For surgical-related disadvantages, the requirement of performing WGL on the day of surgery can create logistical challenges and limit flexibility in surgical scheduling. The wire placement can affect surgical planning and execution. Intraoperatively, difficulty in following the wire’s track or the wire exiting the breast at a distance from the lesion can lead to excessive excision of healthy tissue, thus removing large volumes without achieving improved margins in BCS and affecting the cosmetic outcome (11). The wire can also interfere with optimal positioning of the lesion within the specimen, leading to non-uniform margins.
For patient-related disadvantages, patient discomfort associated with having a wire protruding from the breast can be a significant concern. It may require the use of more than one or two wires, increasing the patient’s discomfort and surgical difficulty. Also, it involves additional radiation for the patient, as the placement of the wire needs to be verified.
As for the cost, although WGL is an economical technique compared to others, one must take into account the cost of the wire material plus the cost of imaging techniques and the increased cost in hospitalization time and surgical time, as radiological verification of the surgical specimen is required (and the operating room minute cost is not negligible) (27).
These issues have driven the development and optimization of WGL technique, as well as the search for alternative localization techniques (28).
Intraoperative ultrasound localization (IOUS)
IOUS localization is a safe, non-invasive technique that involves using US imaging during surgery to locate the lesion (7,29). The IOUS technique is described in a recent article published by the Australasian Society for Ultrasound in Medicine (30). Numerous studies have concluded that IOUS achieves smaller surgical specimen volumes, with a significantly higher proportion of negative margins, resulting in more precise surgery, improved cosmetic outcomes and reduced re-operation risk (31-36). According to two meta-analyses of randomized controlled trials (RCTs), the proportion of positive margins was 5–5.4% and re-operation rate was 4.8–7% (4,7).
Advantages of IOUS
The development of IOUS localization represents a significant step forward in the management of non-palpable breast lesions. Firstly, it is a direct visualization technique performed by the same surgeon intraoperatively, hence there is no need to depend on other specialists. By enabling real-time visualization of the lesion—its size, depth and margin assessment during surgery, it will enable more precise surgical planning and execution. This technique is cost-effective and time-efficient, since no devices need to be placed before surgery, allowing flexibility in the surgical schedule and there’s no need for post-operative specimen verification through radiology. In term of patient benefit, IOUS avoids subjecting the patient to the discomfort of pre-operative needle localization and does not involve the handling of radioactive materials or additional radiation (29).
Limitations of IOUS
The major limitation of IOUS is operator dependency. Ultrasound technique requires training and has a learning curve (37). Another disadvantage is that it requires having an ultrasound machine in the operating room. Furthermore, the application of IOUS is limited to non-palpable lesions that are visible on ultrasound imaging. For non-ultrasound-visible lesions, one can perform IOUS with echo-visible markers like hydrogel clips. Also, deep lesions can be particularly challenging on IOUS, especially if the tumor-to-breast ratio is small. In such circumstances, a low-frequency ultrasound transducer or a longer needle could be helpful (6).
Non-wire localization methods
In this review, the following non-wire localization methods will be discussed—breast biopsy markers, radioactive seeds, magnetic seeds, radar reflectors, and radiofrequency identification tags.
Breast biopsy marker localization
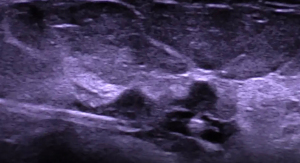
Intraoperative ultrasound-detected marker localization is a method that has been developed to improve the visibility under ultrasound for small or vague lesions (38). US-visible markers are placed after the core needle biopsy for precise breast lesion localization pre-operatively and intraoperatively (39). Apart from US, breast biopsy marker localization can also utilize mammogram, MRI, and CT guidance. Most markers are made of metal (titanium and stainless steel) to generate intense reflection with the ultrasound wave to denote a positive signal. However, sometimes, they can still be masked by calcifications in the breast (40). Non-metal alternatives include carbon-coated ceramic and polyetherketoneketone (PEKK). Subsequent improvements involve markers of various shapes, such as omega and round coil, to make it easier to delineate from body tissue (39). A later generation consists of an additional hydrogel body surrounding the marker: Hydrogel clips (e.g., HydroMARK® and others) (Figure 1) (41). It will expand after insertion into the body to create an additional hypoechoic halo and allow a strong central reflection from the marker (Figure 2) (41).

Advantages of breast biopsy marker localization
HydroMARK® emerges as a marker demonstrating both safety and effectiveness, featuring favorable traits such as a minimal propensity for displacement over time and the ability to remain detectable up to 12 months (42).
The development of marker localization techniques allows for the precise localization of small or vague lesions that may not be easily visualized on ultrasound imaging. Utilizing hydrogel-encapsulated biopsy markers for BCS emerges as a secure and viable substitute, mitigating complications associated with the conventional preoperative WGL approach. This cost-efficient method is poised to enhance the patient’s overall experience and streamline surgical procedures (43,44).
Limitations of breast biopsy marker localization
However, this marker localization technique requires the percutaneous introduction of a foreign body into the breast, which might create potential problems, such as marker extrusion from the skin, challenging placement of marker within a small lesion, and allergic reaction to metallic markers in rare circumstances (45). Besides, metal markers, especially those from the older generation, may not be easily visible and depend heavily on the expertise of the operating surgeon (44).
Radioactive techniques
The concept of using radioactive material for tumor localization was birthed in the late 1990s (46). Initial development and testing were conducted in laboratory settings, using models and animals to evaluate the feasibility and accuracy of the technique.
The radioguided occult lesion localization (ROLL) technique was first introduced by Luini et al. in 1998. It involves injecting a radioactive tracer (technetium-99m colloid) into the lesion under imaging guidance. The tracer emits gamma radiation, which can be detected using a handheld gamma probe during surgery, guiding the surgeon to the lesion (47,48). An advancement of this method is known as sentinel node and occult lesion localization technique (SNOLL). SNOLL involves the concurrent localization of both the hidden lesion and its associated sentinel lymph node using a single-dose radiotracer. This innovative approach enables precise localization of non-palpable breast lesions and simultaneous identification of the sentinel lymph node (49,50).
Additionally, another radioactive technique is radioactive seed localization (RSL). Under imaging guidance, a radioactive iodine 125-labeled titanium-encased seed is implanted into the center of the breast lesion using a needle (51). Intraoperatively, the location of the seed and surrounding lesion are detected by audible feedback from gamma probe and subsequently excised (52). The first clinical trials of RSL were conducted in 2001, demonstrating its potential as an effective alternative to WGL (53). In 2006, iodine-125 was established as one of the safe radioactive markers for locating non-palpable breast lesions (52). When ROLL was compared to RSL, ROLL showed higher patient satisfaction, whilst RSL showed lower proportion of positive margins and lower re-operation rate (4,5).
In 2017, the radioguided occult lesion localisation using iodine-125 seeds (ROLLIS) technique emerged. It is a combination of ROLL and RSL. It utilizes a lower-dose seed and is tested to be safe, effective, and easily applicable in large multidisciplinary environments (54,55). ROLLIS also contributes to patient-centered care, as it was found to reduce their stress and discomfort before surgery when compared to WGL (56).
Recent studies have shown promising results with the use of ROLL for the localization of metastatic axillary lymph nodes as well. For instance, a study by Rella et al. demonstrated that the ROLL procedure for metastatic axillary lymph nodes, identified with a clip marker placement before neoadjuvant chemotherapy initiation, demonstrated an improvement in the detection of residual axillary disease in comparison with sentinel lymph node biopsy alone (57).
The development and adoption of radioactive techniques for the localization of non-palpable breast lesions represent a significant advancement in the field.
Advantages of radioactive techniques
ROLL and ROLLIS are safe, effective techniques, widely studied, and do not require external devices. Furthermore, its greatest advantage is that it allows for the localization of the sentinel lymph node with the same radiotracer (49,50). Radioactive iodine seed has a small size of 4.5 mm and can be placed five days before surgery (58). It also does not have depth limitations for detectability (11). Other advantages include reduction in localization and operation time, lower proportion of positive margins, lower re-operation rate and improved patient comfort compared to WGL (4,5,59).
Limitations of radioactive techniques
However, one of the main challenges is the requirement for close multidisciplinary collaboration between the nuclear medicine and surgical departments, as well as the need for specific equipment and training of new staff due to the radioactive nature of the materials involved (60,61). Furthermore, surgical flexibility could be considered a disadvantage for ROLL since it is necessary to inject the tracer hours or even days before the surgery. Damage of the seed could potentially release radioactive material, with the half life of iodine-125 being 60 days (62,63). Any delay in surgery could put up constructional and logistical challenges, hence, the adoption has been limited (64). Another disadvantage would be the high cost of both the ROLL procedure and the seed. Also, it should be noted that it is not reversible, and it serves as an auditory guide rather than a visual one.
Magnetic techniques
Magnetic seed localization (MSL) is a technique developed in 2016 to overcome some of the limitations of WGL (65). The magnetic seed (Magseed®) (66) (Figure 3) is a small, biocompatible, stainless steel implant that can be inserted into the breast tissue under mammographic or sonographic guidance to mark the location of a lesion (8). The handheld magnetic probe (Sentimag® Localization Platform) (67) (Figure 4) can be used to induce the seed to become a magnet. This is achieved by generating an alternating magnetic field which magnetizes the iron in the seed. The magnetized seed serves as a guide for the surgeon to pinpoint the exact location of the breast lesion (9).


The development of MSL was driven by the need for a more flexible and patient-friendly approach to lesion localization but without the concern about radiation. As mentioned above, traditional WGL required the wire to be inserted on the day of surgery, presenting logistical challenges. US markers, ROLL, and RSL could be alternatives but each has their respective limitations.
The transition of MSL from bench to bedside involved rigorous testing to ensure the safety and effectiveness of the technique. Initial studies were conducted to confirm the magnetic seed’s biocompatibility and determine the seed’s optimal size and magnetic properties for effective localization (68). Following these preclinical studies, clinical trials were conducted to evaluate the use of MSL in patients. These trials demonstrated that MSL was safe and effective, with high successful lesion localization and removal rates (69-73).
Advantages of magnetic techniques
Since receiving Food and Drug Administration (FDA) clearance, MSL has been adopted in many hospitals and clinics. In contrast to WGL and radioactive seed, the small magnetic seed (5 mm) can be inserted any time ahead of surgery and is safe for long-term placement (69,74,75). Clinicians have reported that MSL improves workflow by allowing more flexibility in scheduling. It also avoids the strict protocols and safety precautions associated with RSL due to the lack of radiation concern (65,76). Patients have also reported a positive experience with MSL, with less discomfort and anxiety compared to WGL (77). In a meta-analysis of RCTs published in 2022, patient satisfaction for MSL was 100%, which was the highest compared to WGL, ROLL and RSL (4).
Multiple studies have shown promising results when using magnetic techniques for the localization of non-palpable breast lesions. For instance, a systematic review and pooled analysis by Gera et al. involving over 1,500 lesions demonstrated that the use of magnetic seeds for the localization of non-palpable breast lesions resulted in a high successful placement rate of 94.42% and localization rate of 99.86% (8). Four studies involved in a direct comparison with WGL were included for re-excision rate, which was found to be compatible with no statistically significant difference (18.50% for MSL vs. 16.17% for WGL, P=0.44) (8).
Limitations of magnetic techniques
Despite these potential advantages, magnetic techniques also have their limitations. The main limitation of the Magseed® system is that MRI cannot be used to accurately restage cancer after neoadjuvant chemotherapy. Since the magnetic field generated by the seed or tracer is affected by the magnetic field created by MRI, Magseed®will have a 4–6 cm bloom effect (78,79). Furthermore, due to interactions of magnetic fields between the seed or tracer and other metallic objects in the surgical field, special non-ferromagnetic surgical instruments are required (80). Patients with pacemakers are contraindicated for magnetic localization. Moreover, the device itself is more costly compared to wire and additional training is required for clinical staff (8). Lastly, when multiple Magseed® markers were placed, a distance of 20 mm or greater is required to prevent signal interference between the markers (73).
Radar techniques
Radar techniques involve the use of radar reflectors (80). These devices can be placed inside the breast days or even months before the surgery, thereby avoiding some issues associated with WGL. These markers are introduced percutaneously and identified intraoperatively using a specific probe (81). According to the principles of radar technology, radio waves are transmitted from an antenna to the target lesion. The radar reflector at the site of the lesion reflects radio waves out to the receiving antenna. By calculating the intersections of time of flight between multiple transmitting and receiving antennas, the position of the target can be estimated (82).
One of the systems is called Savi Scout® Surgical Guidance System (Cianna Medical, Aliso Viejo, CA, USA) (Figure 5) (83). This device combines electromagnetic wave technology with infrared light for localization in surgery (80). A handpiece emits electromagnetic waves and non-injurious infrared light, and the electromagnetic wave signal is then reflected from the reflector to provide real-time direction and distance guidance (Figure 6) (83). The radar reflector device contains two antennas made of nickel titanium (6). The detection range can be up to 6 cm deep from the skin surface (Figure 7) (83). Due to its safety profile, the FDA approved it for implantation for an unrestricted length of time (62,84).


Advantages of radar techniques
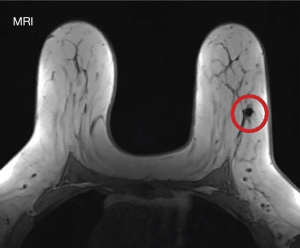
A pilot study for Savi Scout® Localization (SSL) was conducted in 2016 by Cox et al. (81). Subsequently, numerous studies have proven that SSL is safe, feasible, time-efficient, and effective for guiding the excision of non-palpable breast lesions (85-90). This non-radioactive reflector-guided localization technique is favored by both doctors and patients. It has minimal artifacts on MRI (83) (Figure 8), unlike magnetic seed and radiofrequency identification (RFID) tags (6). It overcomes most of the disadvantages of WGL with the absence of external components and the possibility of early placement (81). A recent literature review with pooled analysis showed that SSL has superior outcomes in terms of lower proportion of positive margins and lower re-operation rate compared to WGL, ROLL, RSL, and MSL (5). Furthermore, this innovation is associated with high patient satisfaction (10), minimizing patient discomfort and reducing time delay (62,90). Hence, there is emerging evidence that SSL is a good alternative to WGL that warrants further exploration in our institute.
Limitations of radar techniques
These markers have introduced significant improvements in localizing non-palpable breast lesions. However, certain limitations warrant consideration. The main limitation is the cost, encompassing the radar device itself and associated equipment and training expenses. The system’s learning curve presents an additional challenge, as accurate reflector placement demands training and experience. This could potentially affect localization precision during the initial phases of implementation.
Additionally, it is important to consider its large size (the radar device is the largest of all the devices, measuring 12 mm). This can be an advantage in terms of direct visualization and easy detection but a disadvantage in terms of more challenging placement, especially in the axilla or small breast lesions. The larger size may also lead to more complications, such as hematomas. Furthermore, there may be signal loss and deactivation of the reflector due to surgical electrocautery if the device is damaged during surgery (90). Moreover, patients with implantable devices or nickel allergy are not suitable for the device. There may be weaker signals for deeper breast lesions and hematoma, which are not uncommon after breast biopsy. This could prevent signals from reaching the probe, thus negatively affecting the reflector retrieval (85). If multiple devices are required on the ipsilateral side, the manufacturer’s recommendation is to keep them at least 2.5 cm apart to optimise the distinguishable signals even though it is not an absolute requirement (91).
Despite these limitations, the radar techniques provide a viable option for localization in non-palpable breast diseases. Collaborative efforts among researchers, clinicians, and engineers could drive the development of cost-effective versions, increasing accessibility. Long-term safety studies would provide a comprehensive understanding of its prolonged effects. As the system evolves, it has the potential to address these limitations and further enhance its effectiveness in localizing non-palpable breast lesions.
Radiofrequency-based techniques
RFID technology presents another promising solution for non-palpable breast lesion localization (92). The RFID hand-held reader (interrogator) generates an electromagnetic field that energizes the tag (transponder). The tag receives, alters, and re-emits the radiofrequency signal. Subsequently, the reader captures the altered signal and responds (93,94). The first clinical study to evaluate the safety and performance of this technology was conducted in 2015 (95). One of the RFID systems is called LOCalizer™. This system encompasses two primary components: an RFID tag equipped with a microchip that stores a unique identification number (96) (Figure 9), and a handheld reader that interacts with the tag. This unique number is transmitted via an antenna incorporated within the tag, which responds dynamically to the signals dispatched by the handheld reader (96) (Figure 10). In operation, the tag not only modifies but also retransmits the signal back to the reader, facilitating a response in the form of an audio cue. Moreover, the reader vividly displays the tag’s distinctive identification number along with the precise distance to it, thus potentially streamlining the localization process with a heightened degree of accuracy and efficiency (97).

Advantages of radiofrequency-based techniques
Early experience in the literature showed benefits including easy operability, accessibility due to small handheld single-use relatively low-cost probes that can be used simultaneously by different operation theatres, small probe for more accurate localization, and unique identifier per marker, which allows easy recognition when multiple markers are used (97). Moreover, the RFID tags do not migrate, therefore they are very useful for marking axillary lymph nodes (98). Another study by Lowes et al., one of the largest case series in the literature involving 150 patients, demonstrated its use, including as bracketing tools (92). All tags were successfully retrieved at the surgical site with a re-excision rate of 8.7% only. A systematic review published in 2021 by Tayeh et al. involved 1,151 patients and 1,344 RFID tags. The review concluded that the LOCalizer™ system is a valid, safe, and effective alternative to WGL (99). A recent study by Parisi et al. showed that combined LOCalizer™ and US localization technique had superior oncological outcomes as compared to LOCalizer™ alone (23).
Limitations of radiofrequency-based techniques
Besides the cost, the size of the tag is relatively large (11 mm), even though it is smaller than the radar device (96). The tag generates a 2–5 cm artifact on the MRI (79), which can compromise the assessment, particularly in post-neoadjuvant conditions. Like all non-wire solutions, the tag cannot be further adjusted once deployed. It utilizes an introducer needle that is wider than the introducer needles of Magseed® and Savi Scout®. Therefore, deployment within dense breast tissue could potentially be difficult (99). Furthermore, the RFID system has similar limitations as radar technology, such as dislocation of the marker during insertion, hematoma, and limited detection range (100).
Recent advancements
The integration of augmented reality (AR) in the localization of non-palpable breast lesions represents a current, potentially transformative advancement in breast cancer surgery. Fiber optoacoustic guide (FOG) is a marker that is preoperatively implanted in the tumor; then AR is used for tumor localization and surgical guidance (101). In 2022, a markerless AR localization method using depth sensor and 3D breast CT images was proposed (102). Advantages of AR-guided localization include higher precision in tumor localization, real-time visualization during surgery, thus reducing re-operation rates and shortening the duration of surgery. Although promising research has been published, AR applications in breast surgery are still evolving, and the technology’s maturity needs to be carefully assessed for clinical adoption. Also, AR systems used in medical settings must undergo rigorous certification processes to ensure compliance with medical device regulations (103,104). Limitations of AR localization include lack of interoperability between healthcare systems, video flickering problem intraoperatively (103).
Future development
With the promising new markers, the focus remains on enhancing the patient experience, optimizing oncologic and cosmetic outcomes, in which large trials are still lacking to prove their superiority compared to wire guidance. In addition, knowledge regarding patient-reported outcomes and cosmetic outcomes of different localization methods are still inadequate, therefore future studies in this area would be useful. These could be a major hurdle, especially with their high cost, thus high-quality evidence will be required to justify their use.
On the other hand, advances in nanotechnology and molecular biology could potentially foster the development of more refined, targeted, and less invasive localization methods. Moreover, multidisciplinary approaches encompassing inputs from bioengineers, oncologists, and surgeons are anticipated to facilitate the creation of patient-centric technologies, further tailoring the treatment protocols to individual patient needs and preferences, such as targeted axillary dissection with the help of localization techniques in reducible complications by traditional axillary dissection (105,106). The importance cannot be further stressed with the more popular use of breast screening and improved imaging tools leading to early breast cancer detection. Concurrently, efforts should be channeled towards overcoming the existing barriers to the widespread adoption of newer technologies, including addressing cost-effectiveness and facilitating interdisciplinary collaboration and training.
Conclusions
The evolution of localization methods for non-palpable breast lesions has been characterized by a shift from invasive and uncomfortable procedures to less invasive and more patient-friendly techniques. Each method has its own advantages and limitations, and the choice of method should be individualized based on the characteristics of the lesion, the resources available, and the patient’s preferences. However, there is still room for improvement and innovation in this field. The current gaps in knowledge include the patient-reported outcomes and cosmetic outcomes of different localization methods, therefore future studies in this area would be useful. In addition, the benefits of using biopsy markers, e.g., hydrogel markers, on subsequent surgical outcomes, especially margin status and re-excision rates, are not well documented in the literature, therefore more research on biopsy markers would be needed. Furthermore, research comparing the different non-wire localization techniques is needed, rather than just comparing with wire as the reference technique. Lastly, an area of cutting-edge future research would be on the integration of AR in localization of non-palpable breast lesions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-49/rc
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-49/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-49/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- International WCRF. Worldwide cancer data. London, UK 2023. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: an independent review. Lancet 2012;380:1778-86. [Crossref] [PubMed]
- Haloua MH, Volders JH, Krekel NM, et al. A nationwide pathology study on surgical margins and excision volumes after breast-conserving surgery: There is still much to be gained. Breast 2016;25:14-21. [Crossref] [PubMed]
- Davey MG, O'Donnell JPM, Boland MR, et al. Optimal localization strategies for non-palpable breast cancers -A network meta-analysis of randomized controlled trials. Breast 2022;62:103-13. [Crossref] [PubMed]
- Shirazi S, Hajiesmaeili H, Khosla M, et al. Comparison of Wire and Non-Wire Localisation Techniques in Breast Cancer Surgery: A Review of the Literature with Pooled Analysis. Medicina (Kaunas) 2023;59:1297. [Crossref] [PubMed]
- Guirguis MS, Adrada BE, Scoggins ME, et al. The Challenging Image-Guided Preoperative Breast Localization: A Modality-Based Approach. AJR Am J Roentgenol 2022;218:423-34. [Crossref] [PubMed]
- Banys-Paluchowski M, Kühn T, Masannat Y, et al. Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers (Basel) 2023;15:1173. [Crossref] [PubMed]
- Gera R, Tayeh S, Al-Reefy S, et al. Evolving Role of Magseed in Wireless Localization of Breast Lesions: Systematic Review and Pooled Analysis of 1,559 Procedures. Anticancer Res 2020;40:1809-15. [Crossref] [PubMed]
- Depretto C, Della Pepa G, De Berardinis C, et al. Magnetic Localization of Breast Lesions: A Large-Scale European Evaluation in a National Cancer Institute. Clin Breast Cancer 2023;23:e491-8. [Crossref] [PubMed]
- Tayeh S, Muktar S, Heeney J, et al. Reflector-guided Localization of Non-palpable Breast Lesions: The First Reported European Evaluation of the SAVI SCOUT® System. Anticancer Res 2020;40:3915-24. [Crossref] [PubMed]
- Kapoor MM, Patel MM, Scoggins ME. The Wire and Beyond: Recent Advances in Breast Imaging Preoperative Needle Localization. Radiographics 2019;39:1886-906. [Crossref] [PubMed]
- Catalano O, Fusco R, De Muzio F, et al. Recent Advances in Ultrasound Breast Imaging: From Industry to Clinical Practice. Diagnostics (Basel) 2023;13:980. [Crossref] [PubMed]
- Garzotto F, Comoretto RI, Michieletto S, et al. Preoperative non-palpable breast lesion localization, innovative techniques and clinical outcomes in surgical practice: A systematic review and meta-analysis. Breast 2021;58:93-105. [Crossref] [PubMed]
- Kasem I, Mokbel K. Savi Scout® Radar Localisation of Non-palpable Breast Lesions: Systematic Review and Pooled Analysis of 842 Cases. Anticancer Res 2020;40:3633-43. [Crossref] [PubMed]
- Mendel JB, Long M, Slanetz PJ. CT-guided core needle biopsy of breast lesions visible only on MRI. AJR Am J Roentgenol 2007;189:152-4. [Crossref] [PubMed]
- Lin C, Wang KY, Chen HL, et al. Specimen mammography for intraoperative margin assessment in breast conserving surgery: a meta-analysis. Sci Rep 2022;12:18440. [Crossref] [PubMed]
- Dodd GD, Fry K, Delany W. Preoperative localization of occult carcinoma in the breast. In: Nealon TF, editor. Management of the patient with cancer. Philadelphia: Saunders Co; 1966;183.
- Frank HA, Hall FM, Steer ML. Preoperative localization of nonpalpable breast lesions demonstrated by mammography. N Engl J Med 1976;295:259-60. [Crossref] [PubMed]
- Chadwick DR, Shorthouse AJ. Wire-directed localization biopsy of the breast: an audit of results and analysis of factors influencing therapeutic value in the treatment of breast cancer. Eur J Surg Oncol 1997;23:128-33. [Crossref] [PubMed]
- elzohery YH, Gomaa MM, Mohamed G, et al. Comparison of wire-guided localization in localization of non-palpable breast lesions. World J Surg Onc 2023;21:266
- Ditsch N, Wöcke A, Untch M, et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2022. Breast Care (Basel) 2022;17:403-20. [Crossref] [PubMed]
- Park-Simon TW, Müller V, Jackisch C, et al. Arbeitsgemeinschaft Gynäkologische Onkologie Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2023. Breast Care (Basel) 2023;18:289-305. [Crossref] [PubMed]
- Parisi S, Gambardella C, Conzo G, et al. Advanced Localization Technique for Non-Palpable Breast Cancer: Radiofrequency alone VS Combined Technique with Ultrasound. J Clin Med 2023;12:5076. [Crossref] [PubMed]
- Langen HJ, Kugel H, Grewe S, et al. Preoperative MR-guided localization of suspicious breast lesions. RoFo Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren 2000;172:764-9. [Crossref]
- Kuhl CK, Elevelt A, Leutner CC, et al. Interventional breast MR imaging: clinical use of a stereotactic localization and biopsy device. Radiology 1997;204:667-75. [Crossref] [PubMed]
- Civil YA, Duvivier KM, Perin P, et al. Optimization of Wire-guided Technique With Bracketing Reduces Resection Volumes in Breast-conserving Surgery for Early Breast Cancer. Clin Breast Cancer 2020;20:e749-56. [Crossref] [PubMed]
- Haloua MH, Krekel NM, Coupé VM, et al. Ultrasound-guided surgery for palpable breast cancer is cost-saving: results of a cost-benefit analysis. Breast 2013;22:238-43. [Crossref] [PubMed]
- Tardioli S, Ballesio L, Gigli S, et al. Wire-guided Localization in Non-palpable Breast Cancer: Results from Monocentric Experience. Anticancer Res 2016;36:2423-7. [PubMed]
- Haid A, Knauer M, Dunzinger S, et al. Intra-operative sonography: a valuable aid during breast-conserving surgery for occult breast cancer. Ann Surg Oncol 2007;14:3090-101. [Crossref] [PubMed]
- Khoo FJ, Wong J, Stephenson J, et al. Technique of intraoperative ultrasound-guided excision of impalpable breast lesions. Australas J Ultrasound Med 2023;26:63-6. [Crossref] [PubMed]
- Rahusen FD, Bremers AJ, Fabry HF, et al. Ultrasound-guided lumpectomy of nonpalpable breast cancer versus wire-guided resection: a randomized clinical trial. Ann Surg Oncol 2002;9:994-8. [Crossref] [PubMed]
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systematic review and meta-analysis. Breast Cancer Res Treat 2013;140:435-46. [Crossref] [PubMed]
- Argacha P, Cortadellas T, Acosta J, et al. Comparison of ultrasound guided surgery and radio-guided occult lesions localization (ROLL) for nonpalpable breast cancer excision. Gland Surg 2023;12:1233-41. [Crossref] [PubMed]
- Volders JH, Haloua MH, Krekel NM, et al. Current status of ultrasound-guided surgery in the treatment of breast cancer. World J Clin Oncol 2016;7:44-53. [Crossref] [PubMed]
- Hoffmann J, Marx M, Hengstmann A, et al. Ultrasound-Assisted Tumor Surgery in Breast Cancer - A Prospective, Randomized, Single-Center Study (MAC 001). Ultraschall Med 2019;40:326-32. [Crossref] [PubMed]
- Colakovic N, Zdravkovic D, Skuric Z, et al. Intraoperative ultrasound in breast cancer surgery-from localization of non-palpable tumors to objectively measurable excision. World J Surg Oncol 2018;16:184. [Crossref] [PubMed]
- Esgueva A, Rodríguez-Revuelto R, Espinosa-Bravo M, et al. Learning curves in intraoperative ultrasound guided surgery in breast cancer based on complete breast cancer excision and no need for second surgeries. Eur J Surg Oncol 2019;45:578-83. [Crossref] [PubMed]
- Nurko J, Mancino AT, Whitacre E, et al. Surgical benefits conveyed by biopsy site marking system using ultrasound localization. Am J Surg 2005;190:618-22. [Crossref] [PubMed]
- Alshafeiy TI, Matich A, Rochman CM, et al. Advantages and Challenges of Using Breast Biopsy Markers. J Breast Imaging 2022;4:78-95. [Crossref] [PubMed]
- Blumencranz PW, Ellis D, Barlowe K. Use of hydrogel breast biopsy tissue markers reduces the need for wire localization. Ann Surg Oncol 2014;21:3273-7. [Crossref] [PubMed]
- HydroMARK™ Breast Biopsy Site Markers [Image on the Internet]. Mammotome. Available online: https://www.mammotome.com/us/en/products/breast-biopsy-markers/hydromark
- Sakamoto N, Fukuma E, Tsunoda Y, et al. Evaluation of the dislocation and long-term sonographic detectability of a hydrogel-based breast biopsy site marker. Breast Cancer 2018;25:575-82. [Crossref] [PubMed]
- Gentile LF, Himmler A, Shaw CM, et al. Ultrasound-Guided Segmental Mastectomy and Excisional Biopsy Using Hydrogel-Encapsulated Clip Localization as an Alternative to Wire Localization. Ann Surg Oncol 2016;23:3284-9. [Crossref] [PubMed]
- Chang S, Brooke M, Cureton ERapid Implementation of Intraoperative Ultrasonography to Reduce Wire Localization in The Permanente Medical Group, et al. Perm J 2019;23:18-073. [Crossref] [PubMed]
- Thyssen JP, Menné T. Metal allergy--a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol 2010;23:309-18. [Crossref] [PubMed]
- Dauway EL, Saunders R, Friedland J. Innovative diagnostics for breast cancer: new frontiers for the new millennium using radioactive seed localization. Surgical forum: 85th annual American college of surgeons clinic congress. vol 50 Chicago, IL: The British Institute of Radiology; 1999.
- Luini A, Zurrida S, Paganelli G, et al. Comparison of radioguided excision with wire localization of occult breast lesions. Br J Surg 1999;86:522-5. [Crossref] [PubMed]
- Sajid MS, Parampalli U, Haider Z, et al. Comparison of radioguided occult lesion localization (ROLL) and wire localization for non-palpable breast cancers: a meta-analysis. J Surg Oncol 2012;105:852-8. [Crossref] [PubMed]
- Follacchio GA, Monteleone F, Anibaldi P, et al. A modified sentinel node and occult lesion localization (SNOLL) technique in non-palpable breast cancer: a pilot study. J Exp Clin Cancer Res 2015;34:113. [Crossref] [PubMed]
- Monti S, Galimberti V, Trifiro G, et al. Occult breast lesion localization plus sentinel node biopsy (SNOLL): experience with 959 patients at the European Institute of Oncology. Ann Surg Oncol 2007;14:2928-31. [Crossref] [PubMed]
- Low Activity Radioactive Seeds Used for Localization of Non-Palpable Lesions and Lymph Nodes Licensing Guidance. U.S. Nuclear Regulatory Commission; 2016. Available online: www.nrc.gov/docs/ML1619/ML16197A568.pdf
- Pavlicek W, Walton HA, Karstaedt PJ, et al. Radiation safety with use of I-125 seeds for localization of nonpalpable breast lesions. Acad Radiol 2006;13:909-15. [Crossref] [PubMed]
- Gray RJ, Salud C, Nguyen K, et al. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol 2001;8:711-5. [Crossref] [PubMed]
- Bourke AG, Taylor DB, Westcott E, et al. Iodine-125 seeds to guide removal of impalpable breast lesions: radio-guided occult lesion localization - a pilot study. ANZ J Surg 2017;87:E178-82. [Crossref] [PubMed]
- Brost E, Prather A, Naik H, et al. Use of Reduced Activity Seeds in Breast Radioactive Seed Localization. Health Phys 2020;118:438-42. [Crossref] [PubMed]
- Ong JSL, Teh J, Saunders C, et al. Patient satisfaction with Radioguided Occult Lesion Localisation using iodine-125 seeds ('ROLLIS') versus conventional hookwire localisation. Eur J Surg Oncol 2017;43:2261-269. [Crossref] [PubMed]
- Rella R, Conti M, Bufi E, et al. Selective Axillary Dissection after Neoadjuvant Chemotherapy in Patients with Lymph-Node-Positive Breast Cancer (CLYP Study): The Radio-Guided Occult Lesion Localization Technique for Biopsy-Proven Metastatic Lymph Nodes. Cancers (Basel) 2023;15:2046. [Crossref] [PubMed]
- Ratnagobal S, Taylor D, Bourke AG, et al. Localisation accuracy with iodine-125 seed versus wire guidance for breast cancer surgery. J Med Radiat Sci 2023;70:218-28. [Crossref] [PubMed]
- Ocal K, Dag A, Turkmenoglu O, et al. Radioguided occult lesion localization versus wire-guided localization for non-palpable breast lesions: randomized controlled trial. Clinics (Sao Paulo) 2011;66:1003-7. [Crossref] [PubMed]
- Alikhassi A, Saeed F, Abbasi M, et al. Applicability of Radioguided Occult Lesion Localization for NonPalpable Benign Breast Lesions, Comparison with Wire Localization, a Clinical Trial. Asian Pac J Cancer Prev 2016;17:3185-90. [PubMed]
- Frost R, Reed AJ, Dessauvagie BF, et al. Pre-operative localization of impalpable breast lesions using iodine 125 seeds: Placement accuracy and multidisciplinary challenges. Clin Imaging 2021;73:124-33. [Crossref] [PubMed]
- Farha MJ, Simons J, Kfouri J, et al. SAVI Scout® System for Excision of Non-Palpable Breast Lesions. Am Surg 2023;89:2434-8. [Crossref] [PubMed]
- Radioactive Materials Reference Sheet: Iodine-125: Harvard Campus Services Environmental Health & Safety; 2012. Updated 2012. Available online: https://www.ehs.harvard.edu/sites/default/files/radioactive_materials_reference_sheet_i125.pdf
- Jakub J, Gray R. Starting a Radioactive Seed Localization Program. Ann Surg Oncol 2015;22:3197-202. [Crossref] [PubMed]
- Jeffries DO, Dossett LA, Jorns JM. Localization for Breast Surgery: The Next Generation. Arch Pathol Lab Med 2017;141:1324-9. [Crossref] [PubMed]
- Magseed® MAGNETIC SEED LOCALIZATION [Image on the Internet]. Mammotome; Available online: https://www.mammotome.com/us/en/products/magseed
- Sentimag® Localization Platform [Image on the Internet]. Mammotome; Available online: https://www.mammotome.com/us/en/products/sentimag
- Schermers B, Van Der Hage JA, Van Duijnhoven FH, et al. Magnetic marker localization for non-palpable breast cancer: Initial experience. Eur J Surg Oncol 2016;42:S81. [Crossref]
- Harvey JR, Lim Y, Murphy J, et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat 2018;169:531-6. [Crossref] [PubMed]
- Quinn EM, Dunne E, Stanley E, et al. Magnetic seed localisation for impalpable breast lesions. 44th Sir Peter Freyer Memorial Lecture & Surgical Symposium 2019. Ir J Med Sci 2019;188:S158.
- Thekkinkattil D, Kaushik M, Hoosein MM, et al. A prospective, single-arm, multicentre clinical evaluation of a new localisation technique using non-radioactive Magseeds for surgery of clinically occult breast lesions. Clin Radiol 2019;74:974.e7-974.e11. [Crossref] [PubMed]
- Fung WY, Wong T, Chau CM, et al. Safety and efficacy of magnetic seed localisation of non-palpable breast lesions: pilot study in a Chinese population. Hong Kong Med J 2020;26:500-9. [PubMed]
- Singh P, Scoggins ME, Sahin AA, et al. Effectiveness and Safety of Magseed-localization for Excision of Breast Lesions: A Prospective, Phase IV Trial. Ann Surg Open 2020;1:e008. [Crossref] [PubMed]
- Crèvecoeur J, Jossa V, Di Bella J, et al. Clinical experience of the Magseed(®) magnetic marker to localize non-palpable breast lesions: a cohort study of 100 consecutive cases. Gland Surg 2023;12:566-76. [Crossref] [PubMed]
- Endomag [Internet]. Clinical Information. Indications for Use. Cambridge (UK): Endomag; c2007-2024. 2024. [cited 2024 Apr8]. Available online: https://www.endomag.com/en/indications-for-use/
- Chacko SM, Marshall HN. Implementation of Preoperative Magnetic Seed Localization for Breast and Axillary Lesions: An Alternative to Wires and Radioactive Seeds. J Radiol Nurs 2018;37:154-7. [Crossref]
- Micha AE, Sinnett V, Downey K, et al. Patient and clinician satisfaction and clinical outcomes of Magseed compared with wire-guided localisation for impalpable breast lesions. Breast Cancer 2021;28:196-205. [Crossref] [PubMed]
- Žatecký J, Kubala O, Jelínek P, et al. Magnetic marker localisation in breast cancer surgery. Arch Med Sci 2023;19:122-7. [PubMed]
- Hayes MK. Update on Preoperative Breast Localization. Radiol Clin North Am 2017;55:591-603. [Crossref] [PubMed]
- Mango VL, Wynn RT, Feldman S, et al. Beyond Wires and Seeds: Reflector-guided Breast Lesion Localization and Excision. Radiology 2017;284:365-71. [Crossref] [PubMed]
- Cox CE, Garcia-Henriquez N, Glancy MJ, et al. Pilot Study of a New Nonradioactive Surgical Guidance Technology for Locating Nonpalpable Breast Lesions. Ann Surg Oncol 2016;23:1824-30. [Crossref] [PubMed]
- Adachi M, Nakagawa T, Fujioka T, et al. Feasibility of Portable Microwave Imaging Device for Breast Cancer Detection. Diagnostics (Basel) 2021;12:27. [Crossref] [PubMed]
- MeriMedical. SCOUT® Radar Localization 2023. Available online: https://www.merit.com/product/scout-radar-localization/
- FDA Clears Cianna Medical SAVI Scout Breast Surgical Guidance System [Image on the Internet]. Imaging Technology News; updated 2014 Dec 16. Available online: https://www.itnonline.com/content/fda-clears-cianna-medical-savi-scout-breast-surgical-guidance-system
- Vijayaraghavan GR, Ge C, Lee A, et al. Savi-Scout Radar Localization: Transitioning From the Traditional Wire Localization to Wireless Technology for Surgical Guidance at Lumpectomies. Semin Ultrasound CT MR 2023;44:12-7. [Crossref] [PubMed]
- Bercovici N, Makarenko V, Vijayaraghavan G, et al. A single-institution analysis of reflector-guided localization using SAVI SCOUT® in nonpalpable breast carcinoma compared to traditional wire localization. Breast J 2021;27:737-8. [Crossref] [PubMed]
- Tingen JS, McKinley BP, Rinkliff JM, et al. Savi Scout Radar Localization Versus Wire Localization for Breast Biopsy Regarding Positive Margin, Complication, and Reoperation Rates. Am Surg 2020;86:1029-31. [Crossref] [PubMed]
- Cox CE, Russell S, Prowler V, et al. A Prospective, Single Arm, Multi-site, Clinical Evaluation of a Nonradioactive Surgical Guidance Technology for the Location of Nonpalpable Breast Lesions during Excision. Ann Surg Oncol 2016;23:3168-74. [Crossref] [PubMed]
- Mango V, Ha R, Gomberawalla A, et al. Evaluation of the SAVI SCOUT Surgical Guidance System for Localization and Excision of Nonpalpable Breast Lesions: A Feasibility Study. AJR Am J Roentgenol 2016;207:W69. [Crossref] [PubMed]
- Wazir U, Kasem I, Michell MJ, et al. Reflector-Guided Localisation of Non-Palpable Breast Lesions: A Prospective Evaluation of the SAVI SCOUT(®) System. Cancers (Basel) 2021;13:2409. [Crossref] [PubMed]
- Jadeja PH, Mango V, Patel S, et al. Utilization of multiple SAVI SCOUT surgical guidance system reflectors in the same breast: A single-institution feasibility study. Breast J 2018;24:531-4. [Crossref] [PubMed]
- Lowes S, Bell A, Milligan R, et al. Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: experience of the first 150 cases in a UK breast unit. Clin Radiol 2020;75:942-9. [Crossref] [PubMed]
- Reicher JJ, Reicher MA, Thomas M, et al. Radiofrequency identification tags for preoperative tumor localization: proof of concept. AJR Am J Roentgenol 2008;191:1359-65. [Crossref] [PubMed]
- Lozano-Nieto A. RFID design fundamentals and applications. 1 ed. Boca Raton, FL: CRC Press; 2011.
- Dauphine C, Reicher JJ, Reicher MA, et al. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am J Roentgenol 2015;204:W720-3. [Crossref] [PubMed]
- LOCalizer™ Wire-free Guidance System [Image on the Internet]. Hologic Breast Surgery; [updated 2021. Available online: https://hologicbreastsurgery.com/en/portfolio/localizer-wire-free-guidance-system/
- Malter W, Holtschmidt J, Thangarajah F, et al. First Reported Use of the Faxitron LOCalizer™ Radiofrequency Identification (RFID) System in Europe - A Feasibility Trial, Surgical Guide and Review for Non-palpable Breast Lesions. In Vivo 2019;33:1559-64. [Crossref] [PubMed]
- Lowes S, El Tahir S, Koo S, et al. Pre-operative localisation of axillary lymph nodes using radiofrequency identification (RFID) tags: a feasibility assessment in 75 cases. Clin Radiol 2023;78:e668-75. [Crossref] [PubMed]
- Tayeh S, Wazir U, Mokbel K. The Evolving Role of Radiofrequency Guided Localisation in Breast Surgery: A Systematic Review. Cancers (Basel) 2021;13:4996. [Crossref] [PubMed]
- Christenhusz A, den Dekker BM, van Dalen T, et al. Radiofrequency localization of nonpalpable breast cancer in a multicentre prospective cohort study: feasibility, clinical acceptability, and safety. Breast Cancer Res Treat 2023;201:67-75. [Crossref] [PubMed]
- Lan L, Xia Y, Li R, et al. A fiber optoacoustic guide with augmented reality for precision breast-conserving surgery. Light Sci Appl 2018;7:2. [Crossref] [PubMed]
- Khang S, Park T, Lee J, et al. Computer-Aided Breast Surgery Framework Using a Markerless Augmented Reality Method. Diagnostics (Basel) 2022;12:3123. [Crossref] [PubMed]
- Gouveia PF, Luna R, Fontes F, et al. Augmented Reality in Breast Surgery Education. Breast Care (Basel) 2023;18:182-6. [Crossref] [PubMed]
- Gouveia PF, Costa J, Morgado P, et al. Breast cancer surgery with augmented reality. Breast 2021;56:14-7. [Crossref] [PubMed]
- Weinfurtner RJ, Leon A, Calvert A, et al. Ultrasound-guided radar reflector localization of axillary lymph nodes facilitates targeted axillary dissection. Clin Imaging 2022;90:19-25. [Crossref] [PubMed]
- Gallagher KK, Iles K, Kuzmiak C, et al. Prospective Evaluation of Radar-Localized Reflector-Directed Targeted Axillary Dissection in Node-Positive Breast Cancer Patients after Neoadjuvant Systemic Therapy. J Am Coll Surg 2022;234:538-45. [Crossref] [PubMed]
Cite this article as: Cheung BHH, Co M, Lui TTN, Kwong A. Evolution of localization methods for non-palpable breast lesions: a literature review from a translational medicine perspective. Transl Breast Cancer Res 2024;5:12.


