
The optimal amino acid pattern for humans and its implications for nutrition of cancer patients
Amino acids (AAs) are organic molecules containing both an amino group (-NH2) and an acid group [e.g., carboxylic (-COOH) or sulfonic acid (-SO3H)] (1). AAs occur in nature both freely and bound in peptides or proteins. The simplest AA—glycine—was detected in the coma of the comets 81P/Wild 2 (2) and 67P/Churyumov-Gerasimenko (3), and many more AAs have been found in meteorites (4). Laboratory experiments have shown that AAs, and even whole peptides, can form under conditions resembling those of the cold interstellar medium (5), supporting the notion that these building blocks of life were present on Earth at its very formation. All organisms on Earth, including the most primordial ones, use a ubiquitous genetically encoded set of 20 AAs to synthesize proteins and peptides. These 20 so-called “canonical proteinogenic AAs” (1) appear to have been optimized for building a broad variety of proteins, because they occupy the possible ranges of the three fundamental AA properties size, charge and hydrophobicity more evenly and broadly than other possible AA combinations (6). However, the relative amount of each of these 20 AAs that an individual organism requires for optimizing its physiological functions is species-specific and its estimation relies on approximate empirical methods—until recently, as we will explain in this editorial.
To synthesize a protein, eukaryotic cells first transcribe the corresponding protein-coding DNA region to a pre-mRNA molecule which subsequently undergoes splicing during which specific regions of the mRNA transcript, the introns, are cut out and only the protein-coding regions, the exons, are retained and pasted together (7). The resulting mature mRNA is translocated to the cytosol into ribosomes and translated into protein in a multistep process (1). The totality of exons is known as the exome, which accounts for approximately 1–2% of the eukaryotic DNA, depending on the species.
An organism’s exome thus contains information about the relative proportion with which individual AAs are translated into proteins, providing a logical basis for deducing individual AA requirements for a given species. This has first been tested in a seminal study published in 2017, in which Piper et al. fed fruit flies with an “exome-matched” AA composition (8). This composition was derived in silico as the average relative proportion of each AA based on its prevalence in the entire exome; it differed significantly from the AA composition in natural or laboratory proteins (9). Piper et al. demonstrated that the exome-matched AA diet decreased uric acid production, was more satiating, enhanced growth and increased reproduction compared to a diet containing a non-matched AA composition (8). They also were able to predict which of the so-called essential or indispensable AAs is limiting in a given dietary protein by comparing each of the essential AA’s relative concentration in the dietary protein to the one in the exome-matched protein.
In a recent study published in the Journal of Agricultural and Food Chemistry, a team led by Quingping Wu and Yizhen Xie from the Guangdong Academy of Sciences, China, now derived the exome-matched AA pattern for humans and for the first time tested its application in cancer treatment by using tumor-bearing mice fed with their species-specific exome-matched AA composition (10). Based on approximately 123,000 protein sequences translated from the exome regions for humans and 87,000 for mice, the authors first computed the relative AA composition requirements for each species. The resulting essential and conditionally essential AA pattern for humans was shown to approximately match that recommended by the World Health Organization. A further comparison with soy protein and whey protein isolate—two dietary proteins known to contain all essential AAs for humans—showed that soy protein had limiting amounts of the essential AAs methionine and leucine, while whey protein contained all essential AAs in adequate proportions, thus confirming the superior protein quality of whey protein isolate compared to soy protein and the general possibility to utilize exome-matched AA proportions to evaluate protein quality (10).
The major focus of this study, however, was on the effect of feeding a diet aligned with the exome-matched AA composition to mice inoculated with highly metastatic 4T1 murine breast cancer cells. Mice were divided into four different groups of which all received paclitaxel chemotherapy, but which differed in protein supplementation: one group received a nutritional supplement designed for cancer patients at a dose of 18 g/kg/day; one group received the same supplement at 18 g/kg/day, but adjusted by adding the underrepresented AAs serine and glycine to closely match the AA pattern predicted from the murine exome translation data; one group received the unadjusted supplement but at double dosage (36 g/kg/day); and one group received no nutritional supplement in addition to the standard diet.
First, it is important to mention that additional protein supplementation, independent of composition and dose, did not promote tumor growth compared to the group receiving no protein supplement. However, mice receiving additional protein had higher body mass during the third and final week of the experiment. This justifies clinical guidelines according to which cancer patients should aim at protein intakes in the higher range of 1.5–2 g/kg/day in order to compensate for systemic inflammation and tumor-induced muscle protein degradation, without having to fear that additional AAs could “feed the tumor” (11). In fact, cancer cells are quite independent from dietary AA consumption, because they frequently utilize glutamine as an energetic fuel whose plasma levels are hardly influenced by diet (12); or they cannibalize their host by using a process called macropinocytosis in which whole peptides and proteins are internalized into the cell and subsequently broken down into AAs in order to supply anabolic and catabolic (energy-generating) substrates (13).
A second important observation was that mice receiving the AA-adjusted protein supplement could increase their grip strength significantly more than the mice receiving either no or the unadjusted, glycine- and serine-deficient protein supplement. However, the group receiving the latter at double dosage could increase their grip strength to a similar degree as the mice receiving the adjusted protein formula. Follow-up analyses of the transcriptome profile of skeletal muscle tissue revealed significant differences between the groups receiving the unadjusted and adjusted protein supplement regarding the expression of genes related to immune system modulation within the skeletal muscle microenvironment. In particular, expression of the protein complement 3 (C3) was significantly upregulated in muscle cells from mice having received the adjusted protein supplement. C3 is the central activator of the complement system and has been shown to facilitate muscle regeneration (14). There was also a statistically significant decrease in B cells and increase in monocytes in the skeletal muscle microenvironment of mice receiving the adjusted protein formula, which suggests a link between immune system modulation and increased grip strength development in these mice mediated by receiving an optimal AA pattern.
A third interesting result of the study was that protein supplementation counteracted the paclitaxel-induced gut dysbiosis which consisted in an increased Firmicutes-to-Bacteroidetes ratio in stool samples. Interestingly, mice receiving a double dose of the unadjusted protein supplement had the lowest Firmicutes-to-Bacteroidetes ratio, suggesting that the quantity of AAs may be more important for restoring chemotherapy-induced dysbiosis than AA quality. However, there appeared to be some additional benefits of AA adjustment towards the exome-based AA pattern since the adjusted supplement group had a significantly higher relative abundance of bacteria from the genus Alistipes than the unadjusted supplement group; these bacteria were previously shown to be more prevalent in elderly humans who are physically fit (15), suggesting that protein quality may affect muscle functioning also by modulating the gut microbiome. However, the broader translational implication of this finding is unclear, since currently 13 species of Alistipes are known and some of them may also behave pathogenic in certain contexts (16).
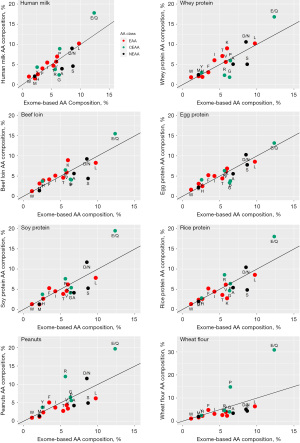
The study by Gong et al. (10) elegantly connects the topic of species-specific AA requirements with translational breast cancer research. It also confirms the notion that protein quality is at least as important as protein quantity, which should be considered when discussing optimal diets for cancer patients. For the first time, the optimal AA pattern for humans has been deduced in a logical way from genomic information rather than measured. The empirical estimation of AA requirements is a difficult task, is not possible for all canonical AAs simultaneously and yields different results, depending on the method (17). This is also reflected in different protein quality metrics, which also vary according to the AA pattern they are compared against (18). In contrast, the exome-based AA pattern calculated by Gong et al. could serve as a new reference against which the quality of different food proteins can be evaluated. This is exemplarily shown in Figure 1, where I have plotted the relative AA composition of various food protein sources against the exome-based AA composition. The quality of a food protein source could be scaled according to the number and extent of indispensable (essential) AAs which fall below the diagonal line in a plot such as the ones displayed in Figure 1. For example, one could use the relative concentration of each essential AA in a given food and in the exome-based AA pattern whose minimum defines the first limiting essential AA (8). Figure 1 shows that proteins of animal origin have less limiting essential AAs than plant proteins, and the deviations of most conditionally essential or nonessential AAs from the ideal AA concentration (the solid line with slope 1) are also smaller. With the exception of soy, most plant proteins are limiting in more than one essential AA and therefore have significantly lower quality than proteins of animal origin. In addition, many edible plants contain phytochemicals known as antinutrients which further reduce protein quality by inhibiting the activity of intestinal proteases and peptidases and thus attenuate the hydrolysis of proteins into absorbable peptides and AAs (21,22). The question of protein quality is highly relevant for cancer patients who often require a higher-than-normal AA supply in order to compensate for AA consumption by their tumors and tumor-induced muscle protein degradation (11). Hence, translating the findings of Gong et al. into clinical practice, it is expected that animal proteins will benefit cancer patients’ skeletal muscle tissue and its immune environment more than equal amounts of plant proteins.
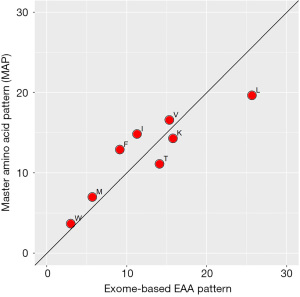
Other clinical innovations that could advance from this study include the provision of exome-based AA supplements to cancer patients or to adjust their dietary proteins to approximately match the exome-based AA pattern by using single AAs. A popular supplement originally called the “master amino acid pattern” (MAP) is available in many countries worldwide and we have used it together with a ketogenic diet in a clinical study of breast cancer patients (23). MAP is only composed of eight essential crystalline AAs (Figure 2) and according to a study by its inventor, Prof. Lucà-Moretti, could be used as the sole dietary protein source with a net nitrogen utilization close to 100% (24). However, while theoretically all nonessential AAs could be synthesized de novo from essential AAs, it has also been shown that in the longer term, diets composed of only the essential AAs are suboptimal for growth, muscle mass acquisition and reproduction (25). In contrast, exactly these traits were shown to be optimized with complete and optimal species-specific AA patterns (8,10,26), so that it would be interesting to compare an exome-based AA supplement against MAP or similar supplements in the future.
What future studies should also investigate is the effect of aligning the dietary AA pattern of patients or healthy subjects with the human exome-based AA pattern. Outcomes of interest would include the effects on muscle strength and transcriptional profiles, immune cells and their cytokines, the microbiome and detoxification pathways, since it would be expected that an optimal AA pattern minimizes the amount of AAs being catabolized and producing nitrogen waste which needs to be detoxified. It would also be interesting to conduct dose-response studies with exome-based AA formulas and find out whether and how gender, age and lifestyle factors such as exercise would affect the intensity of protein transcription, which changes the need for certain AAs, but is not taken into account by the exome-based proportioning of AAs. It is now time to expand the preclinical research on exome-based AA optimization and translate the findings into the clinic.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Breast Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-25/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-25/coif). R.J.K. serves as an unpaid editorial board member of Translational Breast Cancer Research from December 2023 to November 2025. R.J.K. receives royalties from a book on cancer. R.J.K. cooperates with MITOcare GmbH & Co. KG which involves participation in webinars, lectures and the development of nutritional supplements. R.J.K. has also received honoraria from MITOcare GmbH & Co. KG for giving webinars and lectures. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu G. Amino Acids. Biochemistry and Nutrition. 2nd edition. Boca Raton, FL: CRC Press; 2022:816.
- Elsila JE, Glavin DP, Dworkin JP. Cometary glycine detected in samples returned by Stardust. Meteoritics & Planetary Science 2009;44:1323-30. [Crossref]
- Altwegg K, Balsiger H, Bar-Nun A, et al. Prebiotic chemicals-amino acid and phosphorus in the coma of comet 67P/Churyumov-Gerasimenko. Sci Adv 2016;2:e1600285. [Crossref] [PubMed]
- Glavin DP, Dworkin JP, Aubrey A, et al. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteoritics & Planetary Science 2006;41:889-902. [Crossref]
- Krasnokutski SA, Chuang KJ, Jäger C, et al. A pathway to peptides in space through the condensation of atomic carbon. Nat Astron 2022;6:381-6. [Crossref]
- Mayer-Bacon C, Freeland SJ. A broader context for understanding amino acid alphabet optimality. J Theor Biol 2021;520:110661. [Crossref] [PubMed]
- Cole LA. Chapter 9 - DNA Biology: DNA Replication, Transcription, and Translation. In: Cole LA. editor. Biology of Life. 1st edition. London: Academic Press; 2016:55-62.
- Piper MDW, Soultoukis GA, Blanc E, et al. Matching dietary amino acid balance to the in silico-translated exome optimizes growth and reproduction without cost to lifespan. Cell Metab 2017;25:610-21. [Crossref] [PubMed]
- MacArthur MR, Mitchell JR. Feeding the genome: in silico optimization of dietary amino acid composition. Cell Metab 2017;25:486-8. [Crossref] [PubMed]
- Gong C, Jiao C, Liang H, et al. Exome-based amino acid optimization: A dietary strategy to satisfy human nutritional demands and enhance muscle strength in breast tumor mice undergoing chemotherapy. J Agric Food Chem 2024;72:7089-99. [Crossref] [PubMed]
- Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48. [Crossref] [PubMed]
- Seyfried TN, Chinopoulos C. Can the Mitochondrial Metabolic Theory Explain Better the Origin and Management of Cancer than Can the Somatic Mutation Theory? Metabolites 2021;11:572. [Crossref] [PubMed]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013;497:633-7. [Crossref] [PubMed]
- Zhang C, Wang C, Li Y, et al. Complement C3a signaling facilitates skeletal muscle regeneration by regulating monocyte function and trafficking. Nat Commun 2017;8:2078. [Crossref] [PubMed]
- Fielding RA, Reeves AR, Jasuja R, et al. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol 2019;127:110722. [Crossref] [PubMed]
- Parker BJ, Wearsch PA, Veloo ACM, et al. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol 2020;11:906. [Crossref] [PubMed]
- Elango R, Ball RO, Pencharz PB. Recent advances in determining protein and amino acid requirements in humans. Br J Nutr 2012;108:S22-30. [Crossref] [PubMed]
- Adhikari S, Schop M, de Boer IJM, et al. Protein quality in perspective: A review of protein quality metrics and their applications. Nutrients 2022;14:947. [Crossref] [PubMed]
- Morifuji M, Ishizaka M, Baba S, et al. Comparison of different sources and degrees of hydrolysis of dietary protein: effect on plasma amino acids, dipeptides, and insulin responses in human subjects. J Agric Food Chem 2010;58:8788-97. [Crossref] [PubMed]
- Zhang Z, Adelman AS, Rai D, et al. Amino acid profiles in term and preterm human milk through lactation: a systematic review. Nutrients 2013;5:4800-21. [Crossref] [PubMed]
- Cordain L. Cereal Grains: Humanity’s Double-Edged Sword. In: Simopoulos AP, editor. World Review of Nutrition and Dietetics. 1st edition. Basel: Karger; 1999:19-73.
- Sarwar Gilani G, Wu Xiao C, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr 2012;108:S315-32. [Crossref] [PubMed]
- Klement RJ, Champ CE, Kämmerer U, et al. Impact of a ketogenic diet intervention during radiotherapy on body composition: III—final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res 2020;22:94. [Crossref] [PubMed]
- Lucà-Moretti M. A comparative, double-blind, triple crossover net nitrogen utilization study confirms the discovery of the master amino acid pattern. Ann R Natl Acad Med Spain 1998;CXV:397-416.
- Hou Y, Yao K, Yin Y, et al. Endogenous synthesis of amino acids limits growth, lactation, and reproduction in animals. Adv Nutr 2016;7:331-42. [Crossref] [PubMed]
- Hatle JD, Maslikova V, Short CA, et al. Protein storage and reproduction increase in grasshoppers on a diet matched to the amino acids of egg yolk protein. J Exp Biol 2022;225:jeb244450. [Crossref] [PubMed]
Cite this article as: Klement RJ. The optimal amino acid pattern for humans and its implications for nutrition of cancer patients. Transl Breast Cancer Res 2024;5:25.


