
Activin A from primary breast tumors generates a pre-metastatic niche by inducing pulmonary fibrosis
Metastasis is one of the most lethal events associated with tumorigenesis. Tumor cells are shed from a primary tumor mass, as a single cell or in a cluster, into the bloodstream, and are termed circulating tumor cells (CTCs). CTCs then extravasate from the circulation to reside in distant organs. These cancer cells are generally called disseminated tumor cells (DTCs). DTCs frequently die but may remain alive in the dormant (non-proliferative) state in distant organs for many years (Figure 1A). The dormant DTCs occasionally become reactivated and proliferate under certain conditions to form undetected micrometastasis prior to the development of overt macrometastasis (1,2).
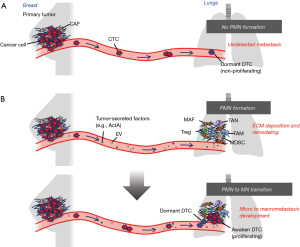
Cancer cells do not exist as isolated entities at the primary tumor and metastatic sites. They actively interact with the tumor microenvironment (TME) composed of non-malignant cells, such as fibroblasts, inflammatory cells, endothelial cells and immune cells that influence tumor hallmarks. Previous studies have documented the existence of a pre-metastatic niche, i.e., the preparation of a microenvironment involving the lungs that facilitates metastatic colonization by DTCs prior to DTC arrival (3,4) (Figure 1B).
A recent elegant study by Cohen et al. published in Cancer Research showed that an increased level of activin A (ActA) released from primary breast cancers induces collagen deposition and fibrosis in the lungs, resulting in the formation of a pre-metastatic niche (5). ActA belongs to the subclass of the transforming growth factor-β (TGF-β) superfamily and is formed from two inhibinβA subunit chains. The expression of ActA is increased in advanced breast cancer and correlates with poor prognosis by promoting epithelial-mesenchymal transition (EMT) and metastasis (6). In the study by Cohen et al., mice harboring a mouse mammary tumor virus-polyoma-middle tumor antigen (MMTV-PyMT) which give rise to spontaneous primary breast tumors and pulmonary metastases, were crossed with Col1a1-YFP transgenic mice, in which Col1a1-expressing fibroblasts had been labeled with YFP (5). Before micrometastases emerged in the lungs, expression levels of profibrogenic genes and collagen deposition were increased in YFP-positive pulmonary fibroblasts, indicating pre-metastatic niche formation. Treatment of normal lung fibroblasts with plasma extracted from MMTV-PyMT mice also induced the production of extracellular matrix (ECM) proteins, such as type I collagen. Elevation of ActA in this plasma component was found to be required for the induction of ECM proteins related to pre-metastatic niche formation.
The pre-metastatic niche, mainly composed of immune cells, inflammatory cells, fibroblasts, endothelial cells and bone marrow-derived cells, serves as the microenvironment enabling DTCs to attach, survive and proliferate to initiate micrometastasis formation. Several immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs), M2-macrophages, regulatory T cells and fibroblasts, also participate in the establishment of an immunosuppressive niche to restrain the anti-tumor responses of CD8+ T cells and natural killer cells. Thus, stromal cells in the pre-metastatic niche have an influential effect on metastasis formation, both directly on cancer cells and indirectly by suppressing tumor immunity.
The primary tumor influences pre-metastatic niche formation through local and systemic immunosuppression. Fibroblasts in healthy lungs produce cyclooxygenase 2 (COX-2), a key enzyme in the biosynthesis of prostaglandin E2 (PGE2), which suppresses immunity by inhibiting dendritic cells and monocytes (7). It is noteworthy that the primary tumor induces the release of IL-1β from lung neutrophils which in turn increases the COX-2-expressing fibroblast proportion in MMTV-PyMT transgenic mouse and syngeneic orthotopic tumor allograft models. The increased PGE2 production in lung fibroblasts then promotes metastasis via immunosuppressive pre-metastatic niche formation. The RNA-binding protein Lin28B was reported to be highly expressed in primary breast tumors and to induce local cytokine milieus in the lung tissues (8). This results in neutrophil recruitment and activation to the N2 phenotype to form an immunosuppressive pre-metastatic niche and promote metastasis. The involvement of tumor-derived ActA in immunosuppression was not, however, addressed in the study by Cohen et al. Whether ActA contributes to immunosuppressive pre-metastatic niche formation merits further investigation.
Soluble factors released from the primary tumor contribute to pre-metastatic niche formation mainly in the lungs. For example, primary tumor-produced vascular endothelial growth factors (VEGFs) induce matrix metalloproteinase-9 (MMP-9) activation and VLA-4/integrin α4β1 expression in endothelial cells, macrophages and hematopoietic progenitor cells in the lungs via VEGF receptor-1 (VEGFR-1)/Flt-1 tyrosine kinase, thereby generating the pre-metastatic niche (3,4). Angiopoietin-2 (ANG-2), a key regulator of angiogenesis, expression is also induced in the vascular endothelium of the lungs by primary tumor-derived VEGFs (9). VEGFs activate calcineurin-nuclear factor of activated T-cells (NFAT) signaling to increase the ANG-2 expression. This initiates angiogenesis in the pre-metastatic niche, which facilitates metastasis. S100A8 and S100A9, produced by murine lung tumor and melanoma cells grown at a primary tumor site, are multifunctional Ca2+ binding proteins that act on myeloid cells as chemokine-like chemoattractant and induce serum amyloid A-3 (SAA3) expression in the lungs (10). The SAA3 then acts on Toll-like receptor 4, a receptor for SAA3 present on myeloid cells, to recruit and activate these cells via NF-κB signaling to establish the lung pre-metastatic niche. Tumor-derived S100A9-positive extracellular vesicles (EVs) also reportedly activated signal transducer and activator of transcription 3 (STAT3) signaling in pulmonary macrophages in a spontaneous lung metastasis mouse model created by orthotopic injection of murine osteosarcoma cells (11). The activated macrophages, in turn, upregulated CXCL2 production to stimulate the recruitment of MDSCs and thereby promoted metastasis in the immunosuppressive environment.
Metabolic reprogramming in stromal cells also contributes to pre-metastatic niche formation by EVs derived from primary breast cancers. MiR-122 enriched in EVs inhibits glucose uptake in brain astrocytes and lung fibroblasts by downregulating expressions of the glucose transporter and pyruvate kinase, a glycolytic enzyme (12). Therefore, the resulting limited competition by stromal cells with decreased glucose uptake leads to upregulation of nutrient availability for DTCs, facilitating metastasis formation.
ECM is mainly composed of proteoglycans and fibrous proteins, including collagen, elastin, fibronectin and laminin. The networks of these components regulate cellular, tissue and organ homeostasis, making the ECM resistant to various mechanical stresses. Periostin (POSTN), and tenascin-C (TN-C) are key ECM proteins which promote metastatic colonization in the lungs. Stromal POSTN recruits Wnt ligands to maintain cancer stemness and promote the formation of pulmonary metastases (13). TN-C derived from breast cancer cells enhances the expressions of stem cell signaling components required, thereby allowing pulmonary micrometastatic colonization (14). Lysyl oxidase (LOX) catalyzes collagen cross-link formation, leading to the generation of strong, thick collagen fibrils. LOX-1, which is a member of the LOX family of proteins, is secreted from primary breast cancer cells via hypoxia-inducible factor-1α-mediated hypoxic responses (15). The secreted LOX-1 accumulates in the lung pre-metastatic niche to crosslink with type IV collagen and recruit CD11b+ myeloid cells. These myeloid cells, in turn, produce MMP-2, which results in type IV collagen remodeling and promotes the recruitment of CTCs, thereby promoting metastatic growth. Type III collagen is produced by cancer cells to induce tumor dormancy by acting on the receptor discoidin domain receptor 1 (DDR1) displayed on these cells both in the primary tumor and at distant sites (16). The downstream STAT1 signaling activation further induces type III collagen expression which maintains tumor dormancy via an autocrine mechanism. In contrast, type III collagen produced by airway smooth muscle cells, which are recruited into the lungs to create a tumor-promoting niche, activates the DDR1-STAT3 signaling in tumor cells to promote metastatic colonization of bladder tumor cells (17). These seemingly contradictory findings reflect the dual roles of type III collagen-DDR1-STAT signaling which is dependent upon the cellular origins of type III collagen.
Carcinoma-associated fibroblasts (CAFs) are a key component of the TME during primary cancer progression. These fibroblasts, via extensive reciprocal signaling interactions, associate with cancer cells. CAFs produce collagen and elastin, which are reoriented and undergo remodeling to generate larger and stiffer fibrils. Fibrosis within the TME facilitates tissue stiffening, which is significantly associated with the progression of breast malignancies (18). CAFs mediate ECM rigidity which in turn promotes metastasis through force- and protease-mediated ECM remodeling. EVs secreted from CAFs in primary salivary adenoid cystic carcinoma induce lung pre-metastatic niche, as exemplified by activated TGF-β-Smad2/3 signaling, POSTN, fibronectin, LOX and MMP-9 expressions, which leads to remodeling of the ECM and promotes metastatic colonization (19).
Stromal fibroblasts present at metastatic sites are referred to as metastasis-associated fibroblasts (MAFs) to distinguish them from CAFs in primary tumors. The transcriptome profiling of MAFs in the lungs of MMTV-PyMT/Col1a1-YFP transgenic mice showed upregulation of Myc signaling which mediated an inflammatory response and ECM remodeling in the lung metastatic microenvironment (20). In MAFs isolated from these mice and breast cancer patients with lung metastases, IL33 expression is robustly upregulated and is involved in the recruitment of T cells and eosinophils into a metastatic niche (21). An investigation employing patient-matched CAFs and MAFs in liver metastases of colorectal cancer obtained evidence that MAFs acquire different gene expression profiles relative to CAFs and have an enhanced ability to promote the progression of cancer hallmarks, such as tumor immunosuppression and therapeutic resistance, due to increased stiffness via ECM remodeling (22). Systemic treatment with doxorubicin after resection of the primary tumor reportedly upregulated the expressions of certain components of the complement system in resident pulmonary fibroblasts in a syngeneic orthotopic mammary tumor mouse model (23). The activated complement signaling was found to promote the mobilization of MDSCs into a metastatic niche, thereby boosting immune suppression and metastatic colonization by DTCs. IL-1 secreted by metastatic breast cancer cells acts on resident fibroblasts in the lungs to induce the CXCL9 and CXCL10 productions via activation of NF-κB signaling (24). CXCL9 and 10, in turn, induce the ability to initiate tumor formation with activated JNK signaling in a small subset of breast cancer cells expressing CXCR3, a receptor for both CXCL9 and 10, giving rise to increased metastatic colonization in the lungs.
The concept of pre-metastatic niche formation in distant organs, a process which favors metastasis from the primary tumor, was first proposed in the early 21st century. Since then, pre-metastatic niche-forming mechanisms have increasingly been uncovered. A better understanding of cellular and molecular mechanisms underlying pre-metastatic niche formation will, it is hoped, provide new insights and lead to novel therapeutic strategies for both the prevention and the treatment of metastatic cancers.
Acknowledgments
Funding: This work was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Breast Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-5/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-5/coif). T.S. and A.O. were supported by JSPS KAKENHI [Grant Numbers JP22K07176 (T.S.), JP24K10459 (A.O.)], a grant from Takeda Science Foundation (T.S.) and from Institute for Environmental & Gender-specific Medicine, Juntendo University (T.S.). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lambert AW, Pattabiraman DR, Weinberg RA. Emerging Biological Principles of Metastasis. Cell 2017;168:670-91. [Crossref] [PubMed]
- Massagué J, Ganesh K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov 2021;11:971-94. [Crossref] [PubMed]
- Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2002;2:289-300. [Crossref] [PubMed]
- Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438:820-7. [Crossref] [PubMed]
- Cohen N, Mundhe D, Deasy SK, et al. Breast Cancer-Secreted Factors Promote Lung Metastasis by Signaling Systemically to Induce a Fibrotic Premetastatic Niche. Cancer Res 2023;83:3354-67. [Crossref] [PubMed]
- Bashir M, Damineni S, Mukherjee G, et al. Activin-A signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer. NPJ Breast Cancer. 2015;1:15007. [Crossref] [PubMed]
- Gong Z, Li Q, Shi J, et al. Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment. Immunity 2022;55:1483-1500.e9. [Crossref] [PubMed]
- Qi M, Xia Y, Wu Y, et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat Commun 2022;13:897. [Crossref] [PubMed]
- Minami T, Jiang S, Schadler K, et al. The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Rep 2013;4:709-23. [Crossref] [PubMed]
- Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol 2008;10:1349-55. [Crossref] [PubMed]
- Deng C, Xu Y, Chen H, et al. Extracellular-vesicle-packaged S100A11 from osteosarcoma cells mediates lung premetastatic niche formation by recruiting gMDSCs. Cell Rep 2024;43:113751. [Crossref] [PubMed]
- Fong MY, Zhou W, Liu L, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 2015;17:183-94. [Crossref] [PubMed]
- Malanchi I, Santamaria-Martínez A, Susanto E, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2011;481:85-9. [Crossref] [PubMed]
- Oskarsson T, Acharyya S, Zhang XH, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 2011;17:867-74. [Crossref] [PubMed]
- Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009;15:35-44. [Crossref] [PubMed]
- Di Martino JS, Nobre AR, Mondal C, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer 2022;3:90-107. [Crossref] [PubMed]
- Lee YC, Kurtova AV, Xiao J, et al. Collagen-rich airway smooth muscle cells are a metastatic niche for tumor colonization in the lung. Nat Commun 2019;10:2131. [Crossref] [PubMed]
- Yoshida GJ, Azuma A, Miura Y, et al. Activated Fibroblast Program Orchestrates Tumor Initiation and Progression; Molecular Mechanisms and the Associated Therapeutic Strategies. Int J Mol Sci 2019;20:2256. [Crossref] [PubMed]
- Kong J, Tian H, Zhang F, et al. Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol Cancer 2019;18:175. [Crossref] [PubMed]
- Shani O, Raz Y, Monteran L, et al. Evolution of fibroblasts in the lung metastatic microenvironment is driven by stage-specific transcriptional plasticity. Elife 2021;10:e60745. [Crossref] [PubMed]
- Shani O, Vorobyov T, Monteran L, et al. Fibroblast-Derived IL33 Facilitates Breast Cancer Metastasis by Modifying the Immune Microenvironment and Driving Type 2 Immunity. Cancer Res 2020;80:5317-29. [Crossref] [PubMed]
- Shen Y, Wang X, Lu J, et al. Reduction of Liver Metastasis Stiffness Improves Response to Bevacizumab in Metastatic Colorectal Cancer. Cancer Cell 2020;37:800-817.e7. [Crossref] [PubMed]
- Monteran L, Ershaid N, Doron H, et al. Chemotherapy-induced complement signaling modulates immunosuppression and metastatic relapse in breast cancer. Nat Commun 2022;13:5797. [Crossref] [PubMed]
- Pein M, Insua-Rodríguez J, Hongu T, et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat Commun 2020;11:1494. [Crossref] [PubMed]
Cite this article as: Shirakihara T, Orimo A. Activin A from primary breast tumors generates a pre-metastatic niche by inducing pulmonary fibrosis. Transl Breast Cancer Res 2024;5:24.


