
A case report of interstitial lung disease caused by HER2-positive breast cancer patient receiving two antibody-drug conjugate drugs successively
Highlight box
Key findings
• For patient, interstitial lung disease (ILD) can be caused with one anti-human epidermal growth factor receptor 2 (anti-HER2) antibody-drug conjugates (ADCs), also can be caused with the following anti-HER2 ADCs.
What is known and what is new?
• Anti-HER2 ADCs can cause ILD. Clinicians need to early detection and intervention to reverse the toxicities and the poor results caused by them.
• How to diagnose and treat ILD be caused with anti-HER2 ADCs has become a new challenge for oncologists. We can refer to “Fudan University Cancer Hospital Standards for the Management of Interstitial Lung Disease Induced by Targeted Drugs in Solid Tumors”. Whether there is a certain relationship between the side effects and efficacy of ADCs, there is no evidence-based data.
What is the implication, and what should change now?
• Whether other anti-HER2 ADCs can be tried as later lines is still being cautious. To clarify the mechanism of ILD caused with anti-HER2 ADCs and the link between the side effects and efficacy of ADCs, we need more cases for studies.
Introduction
According to the 2020 Global Cancer Statistics, the number of new cases of breast cancer has reached 2.26 million, and it has become the first time as the world’s largest cancer (1). With the wide application of molecular typing and classification treatment of breast cancer, comprehensive treatment and individualized treatment have significantly improved the efficacy and survival of breast cancer patients, and the quality of life of advanced patients has also been greatly improved. Comprehensive treatment of breast cancer includes surgery, radiotherapy, chemotherapy, endocrine therapy, targeted therapy and immunotherapy, and the means are extremely rich. With the in-depth understanding of the biological characteristics of breast cancer, especially the discovery of hormone receptor, human epidermal growth factor receptor 2 (HER2) and other driving genes in breast cancer, the diagnosis and treatment of breast cancer has entered the era of molecular therapy and molecular prediction. HER2 is the main driver gene of breast cancer, and its protein amplification or overexpression is an important malignant marker. HER2 positive breast cancer accounts for about 20–25% of all the breast cancers (2). For HER2-positive breast cancer, the application of targeted drugs such as trastuzumab and pertuzumab has significantly improved the survival and prognosis of patients (3). In recent years, small-molecule tyrosine kinase inhibitors (TKIs), antibody-drug conjugates (ADCs) have become one of the significant treatment drugs for patients with HER2-positive advanced breast cancer due to their significant efficacy (4).
ADCs, antibody conjugated drugs, are formed by coupling monoclonal antibodies and small molecule drugs. This drug has the characteristics of high specificity and good safety, and can effectively kill tumor cells. It is clinically used to treat tumor diseases. The mechanism of action of ADCs mainly relies on the targeting effect of monoclonal antibodies to specifically recognize antigens on the surface of tumor cells, and then utilizes the endocytosis of cells in the patient’s body to allow chemical drugs to enter the patient’s tumor cells, achieving the goal of killing tumor cells in the body. However, the ensuing side effects of ADCs can not be ignored. ADC cause some common side effects, such as nausea, vomiting, diarrhea, and bone marrow suppression. ADCs also cause some rare but serious side effects. Some ADCs may cause myocardial damage or heart muscle dysfunction, and in severe cases, may even lead to heart failure. In addition, other serious adverse events (AEs) include pulmonary toxicity, liver toxicity, renal toxicity, etc. In DESTINY-Breast01 study, the investigators reported 20 cases of interstitial lung disease (ILD), including one grade 3 event and two treatment-related deaths due to pneumonia (5). It can be seen that the incidence and mortality of ILD need to be concerned by clinicians.
ILD is a general term for a group of diffuse lung diseases. Experts from the respiratory department, oncology department, imaging department and other departments have reached a consensus on the management of lung injury related to antineoplastic drugs on the diagnosis and treatment of ILD. We are required to first identify early and provide sufficient information and notification to patients, informing them of the potential risk of ILD when administering medication; informing patients that they should promptly communicate with their doctors when experiencing fever, cough, difficulty breathing, or newly emerging or worsening respiratory symptoms. Doctors should also closely monitor the patient’s condition after administering ADC. High resolution chest computed tomography (CT) is an important tool for evaluating ILD and follow-up observation. In addition, bronchoscopic bronchoalveolar lavage and pathological biopsy can also be performed as appropriate for differential diagnosis to exclude infectious ILD. Our case is an advanced HER2 positive breast cancer. After relapse, it received two times of ADCs treatment, ILD has occurred during the treatment period. We present this article in accordance with the CARE reporting checklist (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-19/rc).
Case presentation
Clinical history
A 46-year-old female, who found a left breast mass in January 2020. A biopsy of the left breast mass was performed. The pathological examination showed invasive ductal carcinoma. The immunohistochemical (IHC) test results were estrogen receptor (ER) negative (<1%), progesterone receptor (PR) negative, HER2 (2+ to 3+) and Ki-67 (30%). The HER2 fluorescence in situ hybridization (FISH) detection is HER2 amplification. The clinical stage was cT1N1M0 IIA (HER2-positive type).
The patient received three cycles of neoadjuvant chemotherapy with TEC, docetaxel (75 mg/m2, d1), epirubicin (75 mg/m2, d1), and cyclophosphamide (500 mg/m2, d1), intravenous injection, every 3 weeks. The patient’s tumor efficacy evaluation using RECIST v1.1 showed stable disease (SD). Subsequently, the patient underwent four cycles of THP, docetaxel (75 mg/m2, d1), trastuzumab (the first dose of trastuzumab was 8 mg/kg, and the subsequent dose was 6 mg/kg, d1), and pertuzumab (the first dose of pertuzumab was 840 mg, and the subsequent dose was 420 mg, d1), intravenous injection, every 3 weeks. After four cycles of THP, the patient’s left breast tumor was evaluated as partial response (PR). Then on June 18, 2020, the patient underwent a modified radical mastectomy. The pathological report was invasive ductal carcinoma, grade II, focal ductal carcinoma in situ (DCIS), and the tumor diameter was 0.6 cm × 0.5 cm. The Miller & Payne (MP) Grading System: G3. Non metastasis in sentinel lymph node (SLN): 0/1. Tumor-node-metastasis (TNM) stage: ypT1bN0M0. The IHC test were ER(−), PR (1%), HER2 (3+) and Ki-67 (40%). The patient does not need to receive postoperative adjuvant radiotherapy, and then continued to receiving adjuvant treatment with trastuzumab plus pertuzumab until completion for 1 year.
In April 2021, the patient found enlarged lymph nodes in the left supraclavicular region, and underwent lymph node core biopsy. The pathological examination confirms invasive ductal carcinoma. IHC test were ER(−), PR (−), HER2 (3+) and Ki-67 (40%). Due to the patient’s diagnosis of first-time recurrence and metastasis, we conducted a comprehensive clinical evaluation. The comprehensive clinical evaluation and imaging scanning of the head, chest and upper abdomen, the results were normal. Patient received pyrotinib plus capecitabine as first-line therapy from April 2021 to May 2022. The regimen was pyrotinib (400 mg, oral, qd) and capecitabine (1,500 mg, oral, bid, 2 weeks on and 1 week off). The patient experienced diarrhea (grade 2), stomatitis (grade 1) and hand foot syndrome (grade 2) during medication. She also reduced her dosage to 320 mg/d due to diarrhea. The patient undergoes tumor efficacy evaluation every 2–3 months, which is assessed as PR. Until May 2022, the patient once again discovered an enlargement of the left supraclavicular lymph nodes, which confirmed by re-biopsy. The progression-free survival (PFS) was 13 months.
The pathological examination confirms that it was poorly differentiated adenocarcinoma (metastases from breast cancer). The IHC results were ER(−), PR(−), HER2 (2+ to 3+), Ki-67 (5–10%). Patient received inetetamab plus vinorelbine as second-line therapy from June 2022 to August 2022. The regimen was inetetamab (520 mg, iv, d1) plus vinorelbine softgels (90 mg, oral, d1, 8) for 3 weeks repeat. The evaluation of tumor efficacy was progressive disease (PD) after three cycles and the PFS was 2 months.
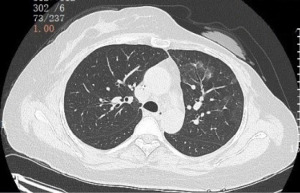
Due to the patient’s progression after undergoing treatment regimens with anti-HER2 monoclonal antibodies and small molecule TKIs, according to the guidelines at the time, ADCs was the preferred option. On September 30, 2022, the patient participated in a clinical trial of an ADC in The Fifth Medical Center of PLA General Hospital as third-line therapy, and was treated with ARX-788 (98 mg, iv, d1, q3w). After four cycles of treatment, the tumor evaluation was PR. After six cycles (February 20, 2023), the patient was excluded from the clinical study because of ILD (Figure 1). The patient was given systemic prednisolone regimen, prednisolone 0.5 mg/kg/day is recommended until improvement, followed by gradual tapering over 4 weeks. After 5 weeks of prednisolone therapy, the CT scan of the chest showed that ILD improved significantly (Figure 2). On March 30, 2023, the patient received anti-HER2 therapy with trastuzumab-DM1 (T-DM1) as fourth-line therapy, the specific dose was: T-DM1 200 mg d1 q3w. On April 20, 2023, when the patient came back the hospital for the second cycle of T-DM1, the patient reported slightly worsening shortness of breath, which became more pronounced during the process of walking stairs. Therefore, we gave her a chest CT scan and found that her ILD reappeared (Figure 3). Although the patient’s symptoms were not obvious, we decided to terminate anti-tumor treatment and continue to treat ILD with systemic corticosteroids. The corticosteroids were discontinued after 6 weeks of corticosteroid therapy for ILD. As of the last follow-up in April 2024, the tumor was evaluated as SD for more than 12 months (Figure 4 is the timeline of the patient’s treatments).



All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Different anti-tumor drugs have significant differences in efficacy, due to their different mechanisms. Traditional cytotoxic drugs, while achieving therapeutic effects, are accompanied by serious toxic side effects, and often reducing dosage or delaying the regimens. Molecular targeted drugs often have definite efficacy, relatively tolerable toxicity, and even only mild toxicity. ADCs, as a new type of antibody conjugated anti-tumor drug, are known as “magic bullets”. They have high anti-tumor efficacy, even miraculous effects, but their toxicity is relatively controllable. We found that ILD caused by anti-tumor drugs is very common. So far, thousands or even hundreds of drugs have been found to be associated with pulmonary toxicity (6). Among them, anticancer drugs are one of the most common factors leading to ILD (7). Although ADC is a magic anti-tumor drug in the field of breast cancer (8), the clinicians still have little experience in managing drug toxicities. Especially, it was found that the incidence of ILD in some ADCs reached 1.9–15.8% (7).
ILD is a general term for a group of clinical-pathological entities with diffuse exudation, infiltration and/or fibrosis of the lung interstitium as the main lesions, and its clinical symptoms include active dyspnea, diffuse infiltrate opacity on chest X-ray, restrictive ventilation disorder, decreased diffusion function and hypoxemia (9). Due to its diverse clinical symptoms and the lack of specific diagnostic criteria, drug-induced interstitial lung disease (DILD) is difficult to diagnose (7).
There are multiple methods and tests available to assess the severity of the condition, including diagnostic tests such as blood oxygen saturation (SpO2), Krebs von den Lungen-6 (KL-6), and surfactant protein D (SPD). SpO2 is an important physiological parameter that reflects the oxygenation status of patients and is of great significance for evaluating lung function in ILD patients. Serum KL-6 is a serum biomarker associated with ILD related to connective tissue disease, and its level can reflect the activity and progression of the disease. SPD and other diagnostic tests may involve other aspects of examination to comprehensively evaluate the patient’s condition. However, due to the limited experimental conditions in hospitals, KL-6 and SPD are not included as routine testing items.
Although surgical lung biopsy (SLB) is a classic diagnostic method for ILD, it cannot be routinely performed by general medical institutions due to its certain risks and the need for the relative strength of thoracic or pulmonary surgery in medical institutions. The most important for accurate diagnosis is the exclusion of other causes of lung damage. A timely and accurate diagnosis of DILD is important for the treatment and prognosis of patients (10). If not treated properly, it can even be fatal.
ADC are conjugated from antibody drugs targeting particular antigens and payloads (small molecule cytotoxic drugs) through linkers, which have the powerful killing effect of traditional small molecule cytotoxic drugs and the tumor targeting of antibody drugs (11). ADCs are composed of three parts: antibody drugs targeting specific antigens, payloads, and linkers, among them payload is an important part of the anti-tumor effectiveness of ADCs (12).
The therapeutic mechanism of ARX-788 and T-DM1 used in this patient has been well clear, the payload of ARX-788 is a cytotoxic tubulin inhibitor, amberstatin 269 (AS269) (13). ARX-788 utilizes a unique unnatural amino acid site-specific coupling technology to introduce para-acetylphenylalanine (pAF) into HER2 monoclonal antibody, which is site-directed conjugated to the cytotoxic tubulin inhibitor AS269 to form a stable oxime bond. Upon endocytosis into cells, ARX-788 releases pAF-AS269 in response to lysosomes, which induce cell death by binding to microtubules (14). The payload of T-DM1 is emtansine (DM1). T-DM1 is guided by trastuzumab to the vicinity of HER2-overexpressing tumor cells, binds to the C-terminus of HER2 extracellular receptor domain IV, and stimulates complex endocytosis into cells. After internalization, linkers are cleaved in lysosomes, partially hydrolyzing the antibody into Lys-MCC-DM1 with lysine acid, which binds to tubulin, arrests cell split, and ultimately causes cell death (14).
However, the mechanism of ARX-788 and T-DM1 causing ILD has not yet been determined. At present, the pathogenesis of drug-induced ILD is mainly recognized as follows: (I) targeted independent uptake of ADC, cytotoxic drugs may directly damage the type I epithelial, vascular endothelium cells, or airway epithelial cells. (II) The drug may act as a hapten or mimic a host antigen to activate immune cells, resulting in a series of immunogenic reactions (15). The unconjugation of ADC causes the payload to enter the bloodstream. The ILD caused by ADC is mostly reported in HER2 targets, and the exact mechanism is still unclear (16).
Risk factors associated with ILD include older age, male, previous or present smoking history, prior history of ILD, short time to diagnosis of cancer (<6 months), chemoradiotherapy recently, pulmonary infection, chronic obstructive pulmonary disease, and other structural lung diseases; less than 50% normal lung tissue on imaging (17,18). This patient had no risk factors associated with ILD, but was treated with ARX-788, T-DM1 and then all of them had the toxicity and side effect of ILD. At the same time, this patient had occult ILD and had no clinical symptoms such as cough and shortness of breath, which were found during the re-examination and evaluation. It is easy to continue anti-HER2 ADC therapy without imaging prompts, which will resulting in further aggravation of ILD and even irreversible damage. The ILD caused by ADCs varies significantly depending on the different drugs used. Some ADCs, such as sacituzumab govitecan (SG), almost do not cause ILD. However, once a patient develops ILD due to ADC, the risk of recurrence increases significantly when using the same or similar ADCs again, which should be given clinical attention. Especially ARX788 and T-DM1 carry similar toxins, anti-microtubule derivatives of maytansine.
In 2021, Zhang’s team from Fudan University Cancer Hospital formulated the “Fudan University Cancer Hospital Standards for the Management of Interstitial Lung Disease Induced by Targeted Drugs in Solid Tumors”. It is recommended that patients with respiratory symptoms such as cough, sputum production, fever, chest pain, shortness of breath or dyspnea should complete relevant examinations, and chest CT is recommended for imaging. Laboratory testing should include complete blood count detection, C-reactive protein (CRP) detection, procalcitonin (PCT) detection, tuberculosis-infected T cell (CT-SPOT) detection, G test, blood culture, etc. Based on the above examination results, pathogenic infectious pneumonia should be ruled out first. It is important to rule out mycobacterium tuberculosis and fungal infections, as these two diseases are not suitable for high-dose corticosteroid therapy. Once the patient develops grade 1 ILD, the antineoplastic therapy should be discontinued, and we must immediately provide standardized and systemic prednisolone regimen, 0.5 mg/(kg·d) or equivalent doses. Chest CT is re-examined within 1–2 weeks, and the Based on the symptoms and signs within 4 weeks after the start of corticosteroids and the improvement of chest CT, the outcome of ILD should be determined. Once ILD improves, the dose of corticosteroids should be gradually reduced, and the reduction process must be greater than or equal to 4 weeks. Only the ILD recovers to grade 0 for less than 4 weeks, the original dose is administered (19).
Although the patient in this case had obvious symptoms after treatment with glucocorticoids and no coughing or shortness of breath, their chest CT scan still showed grade 1 and did not recover to grade 0. Due to concerns about the risk of tumor progression caused by prolonged discontinuation of anti-tumor drugs, another type of anti-HER2 ADC, T-DM1 was subsequently used based on the patient’s wishes. Unfortunately, the patient only underwent two cycles of T-DM1 treatment, and their ILD worsened.
For this patient who have been effectively treated by ADC for six courses of treatment, if the toxic and side effects have not been completely recovered, they can not rush to give the original anti-tumor drug treatment again, especially ADCs, because most tumors will stabilize for a long time in this case. On the contrary, rushing to take medication may backfire. We should attach great importance to the clinical management of ILD caused by ADC, and relevant guidelines and consensus should be strictly followed to be highly vigilant about the occurrence of ILD. Regular evaluation, close monitoring, standardized treatment, and hormone therapy should be sufficient and complete. If ADC needs to be used again, and the pros and cons should be weighed.
Conclusions
Based on the treatment process and outcome of this case, we suggest that for patients with HER2 positive advanced breast cancer who have been effectively treated by ADC, if there are serious toxic side effects, such as ILD, we should focus on actively treating ILD according to standards, and ensure sufficient corticosteroids and sufficient treatment duration. Once the disease has improved significantly, the corticosteroids dosage can be gradually reduced until the ILD grading was 0–1.
With the widespread application of ADC, clinical doctors are gradually paying attention to the side effects of ADC, among which asymptomatic ILD is easily overlooked due to its concealment. Once delayed in diagnosis and treatment, it can easily lead to serious consequences, even endangering life. Therefore, clinical doctors should closely monitor, intervene in a timely manner, provide sufficient glucocorticoid treatment, and standardize dosage reduction to minimize the harm of ILD and ensure patient safety.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-19/rc
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-19/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-24-19/coif). X.W. serves as an unpaid editorial board member of Translational Breast Cancer Research from December 2022 to November 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer 2021;7:56. [Crossref] [PubMed]
- Marczyk VR, Rosa DD, Maia AL, et al. Overall Survival for HER2-Positive Breast Cancer Patients in the HER2-Targeted Era: Evidence From a Population-Based Study. Clin Breast Cancer 2022;22:418-23. [Crossref] [PubMed]
- Song Y, Lv Y. Antibody-drug conjugate monotherapy refines the oncological efficacy as compared to therapy of physicians’ choices in advanced breast cancers: a systematic review and meta-analysis. Transl Breast Cancer Res 2023;4:11. [Crossref] [PubMed]
- Tamura K, Tsurutani J, Takahashi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol 2019;20:816-26. [Crossref] [PubMed]
- Skeoch S, Weatherley N, Swift AJ, et al. Drug-Induced Interstitial Lung Disease: A Systematic Review. J Clin Med 2018;7:356. [Crossref] [PubMed]
- Dai HP, Ma F, Ren YH, et al. Expert Consensus on the Diagnosis and Treatment of Anticancer Drug-Induced Interstitial Lung Disease. Curr Med Sci 2023;43:1-12. [Crossref] [PubMed]
- Xu Y, Zhang L, Qiao X, et al. Efficacy and safety of antibody-drug conjugates in the treatment of breast cancer: a meta-analysis. China Pharmacy 2023;34:2540-4.
- Wu Q, Guo MN. Classification of interstitial pneumonia. Medical Recapitulate 2001;7:300-1.
- Camus P, Fanton A, Bonniaud P, et al. Interstitial lung disease induced by drugs and radiation. Respiration 2004;71:301-26. [Crossref] [PubMed]
- Liu WC, Li HF, Hu CH. Technology Advancement in Development of Antibody Drug Conjugates. Progress In Biochemistry and Biophysics 2023;50:1167-89.
- Gao L, Wang Y, Wang Y, et al. General considerations for clinical pharmacology of antitumor antibody-conjugated drugs: Implications from FDA review cases. Chinese Journal of Clinical Pharmacology and Therapeutics 2023;28:75-85.
- Skidmore L, Sakamuri S, Knudsen NA, et al. ARX788, a Site-specific Anti-HER2 Antibody-Drug Conjugate, Demonstrates Potent and Selective Activity in HER2-low and T-DM1-resistant Breast and Gastric Cancers. Mol Cancer Ther 2020;19:1833-43. [Crossref] [PubMed]
- Chen K, Huang Y, Wang X, et al. Clinical application and research progress of antibody drugs conjugation in breast cancer. Chinese Journal of Clinical Pharmacology and Therapeutics 2023;28:898-909.
- Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 2012;13:39. [Crossref] [PubMed]
- Hackshaw MD, Danysh HE, Singh J, et al. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2020;183:23-39. [Crossref] [PubMed]
- Long K, Suresh K. Pulmonary toxicity of systemic lung cancer therapy. Respirology 2020;25:72-9. [Crossref] [PubMed]
- Suh CH, Park HS, Kim KW, et al. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients: Meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer 2018;123:60-9. [Crossref] [PubMed]
- Zhang J, Shen WN, Ji DM, et al. FUSCC criteria for the management of targeted drug-induced interstitial lung disease in solid tumors. China Oncol 2021;31:241-9.
Cite this article as: Xie W, Wang T, Wang X. A case report of interstitial lung disease caused by HER2-positive breast cancer patient receiving two antibody-drug conjugate drugs successively. Transl Breast Cancer Res 2024;5:36.


