
Modern approaches to lymphatic surgery: a narrative review
Introduction
Lymphedema is characterized by increased lymphatic fluid in the interstitial spaces due to damage of the lymphatic system. Through a chronic inflammatory process of lymphosclerosis and fat deposition, clinical characteristics of pitting edema secondary to excess fluid progress to non-pitting edema, excess fibroadipose tissue and skin changes (1,2). Primary, or idiopathic, lymphedema refers to the malformation of the lymphatic system and can be present at birth (i.e., congenital lymphedema) or develop later in life (3). Secondary lymphedema refers to the acquired factors impacting the function of a normally developed lymphatic system. Oncologic extirpation, notably axillary lymph node dissection (ALND), is the most common cause of secondary lymphedema in developed countries, but other causes include trauma, infection and radiation (4,5). The overall incidence of lymphedema is complex as it is often underrecognized and undertreated, but estimates indicate primary lymphedema affects 1 in 100,000 people, and secondary lymphedema afflicts 1 in 1,000 Americans (6).
Lymphedema symptoms including chronic swelling and enlargement of an extremity impair activities of daily life, quality of life, and can result in recalcitrant wounds and infections when severe. Non-surgical treatment of lymphedema is known as complete decongestive therapy (CDT), a multimodal staged treatment regimen involving compression garments, bandaging, manual lymphatic drainage, physical therapy exercises, and hygiene care.
Surgical options can be categorized as physiologic or reductive procedures. While neither approach is curative, both aim to provide patients with symptom relief. Physiologic procedures target the fluid-dominant portion of the disease while reductive procedures address the late-stage, fibroadipose pathology of lymphedema. Physiologic approaches include lymphovenous bypass (LVB) and vascularized lymph node transplant (VLNT), and reductive techniques include liposuction and excisional surgery. LVB is a supermicrosurgical technique that effectively shunts lymphatic drainage into the systemic circulation distally by connecting lymphatic vessels to subdermal venules. VLNT is another physiologic approach wherein an autologous free flap containing a cluster of lymph nodes on a vascularized pedicle is relocated to the lymphedematous extremity. For patients with an additional fibroadipose component of disease, debulking surgery as part of their overall treatment plan should be considered for optimal outcomes (7). These encompass liposuction as well as excisional surgeries such as the Charles and Homan’s procedures which aim to remove excess fibroadipose tissue (8-10).
Given this knowledge in the literature, this review focuses on current approaches to lymphedema treatment including modern imaging technologies, contemporary surgical approaches, and future directions for the field of lymphatic surgery. We present this article in accordance with the Narrative Review reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-49/rc).
Methods
A systematic review of PubMed, Embase, and Cochrane Library databases was used to identify approaches to surgical management of lymphedema from January 2000 to February 2024. MeSH terms and keywords included “lymphovenous bypass surgery”, “lymphovenous bypass”, “lymphovenous anastomosis”, “lymphovenous anastomosis surgery”, “lymphatic surgery”, “lymphedema surgery”, “vascularized lymph node transfer”, “vascularized lymph node transplant”, “physiologic lymphedema surgery”, “excisional or reductive lymphedema surgery”, “vascularized lymphatic vessel transplant”, “vascularized lymphatic vessel transfer”, “VLVT”, and/or “imaging for lymphedema”. Inclusion criteria included observational articles including retrospective and prospective studies, experimental original articles, systematic reviews, and meta-analyses. Non-English manuscripts and abstracts were excluded from the review (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | February 29, 2024 |
| Databases and other sources searched | PubMed, Embase, Cochrane Library |
| Search terms used | “Lymphovenous bypass surgery”, “lymphovenous bypass”, “lymphovenous anastomosis”, “lymphovenous anastomosis surgery”, “lymphatic surgery”, “lymphedema surgery”, “vascularized lymph node transfer”, “vascularized lymph node transplant”, “physiologic lymphedema surgery," “excisional or reductive lymphedema surgery”, “vascularized lymphatic vessel transplant”, “vascularized lymphatic vessel transfer”, “VLVT”, and/or “imaging for lymphedema” |
| Timeframe | 1/1/2000–2/29/2024 |
| Inclusion and exclusion criteria | Inclusion: all studies including case reports, case series, randomized control trials, literature reviews |
| Exclusion: studies not written in English | |
| Selection process | E.Z., N.Y. conducted the study independently and consensus was obtained with review from all authors |
Discussion
Immediate lymphatic reconstruction (ILR)
ILR is a prophylactic surgery considered for populations at increased risk of developing secondary lymphedema, and is performed at the time of lymphadenectomy. In 2009, Boccardo et al. described their findings in the Lymphedema Microsurgical Preventative Healing Approach (LyMPHA) study which involved performing an axillary LVB immediately following ALND in 19 patients (11). They found that LVB prevented secondary lymphedema in all cases at both follow-up periods of 6 and 12 months. Variations of this technique have become the mainstay for ILR which typically utilize telescoping or intussusception anastomosis of cut lymphatic channels draining the upper extremity to branches of the axillary vein (Figure 1). A follow-up study over four years later demonstrated a 4% rate of lymphedema (11). Subsequent similar studies of prophylactic ILR following ALND have described a lowered rate of lymphedema ranging from 4% to 12% (12-15). More recently, the preliminary results of a randomized controlled trial comparing ILR to no lymphatic reconstruction following ALND for breast cancer demonstrated an incidence of breast cancer related lymphedema (BCRL) in 9.5% of the ILR group compared to 32% in the control group at 24 months (16). Secondary outcomes additionally supported this finding with lower bioimpedance values, less dermal backflow, and improved quality of life in the ILR group.
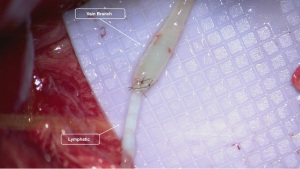
In cases where there is considerable distance between the afferent lymphatic and the target vein, a vein graft may be utilized (17,18). The use of vein grafts allows for more flexibility in choosing the final recipient vein, incorporation of valves to minimize backflow, improved size matches, and facilitation of multiple independent anastomoses with different branching patterns. The more routine use of vein grafts has led certain authors to decrease abortion rates from 14% to 0% in ILR (17). Venous couplers have also been described for LVB during ILR (19,20). Spoer et al. described a coupler-assisted technique anastomosing multiple lymphatics to a single vein (19). Other authors have also utilized couplers in an end-to-end fashion during immediate LVB with good short-term reported outcomes (20). Prophylactic VLNT for ILR has also been described though is less commonly utilized (21). These procedures are typically reserved for patients with severe anticipated soft tissue loss after extirpative nodal surgery and subsequent radiation.
Intraoperative mapping of lymphatic channels is a critical component of ILR. The use of blue dye and indocyanine green (ICG) are commonly utilized through intradermal injection in the ipsilateral extremity to identify lymphatics cut during ALND that are draining the upper extremity. More recently, fluorescein isothiocyanate (FITC) has been described to aid in identification of lymphatics during active dissection (22). As FITC is excitable in the visible spectrum, it is possible to view the fluorescence using a fluorescent-capable microscope amongst the surrounding tissues in one visual surgical field (22).
ILR has also been described in the lower extremity. Alarcón et al. utilized both LVB and VLNT for lower extremity ILR with a lymphedema rate of 10.5% in the prophylactic cohort compared to 37% in the retrospective control group without lymphatic reconstruction (23). Cakmakoglu et al. employed ILR for patients undergoing ilioinguinal lymphadenectomy, and amongst the 12 patients, none developed lymphedema (24). Promising results have also been demonstrated in gynecological neoplasms. In seven patients with uterine neoplasms underdoing hystero-oophorectomy and intrapelvic lymph node dissection, Takeishi et al. performed prophylactic intrapelvic LVB and found 1 of 7 patients to have mild lymphedema in a follow-up period of 18 months (25). Comparative studies on LVB at the time of inguinal lymph node dissection identified an incidence of lymphedema at 8.3% with ILR compared to 25.0% for the historical control (26). While ILR has been described at the time of melanoma resection (27), general consensus guidelines have advised against prophylactic ILR at the time of resection of cutaneous extremity neoplasms (28).
While immediate, proximally-based LVB is the leading surgical technique for lymphedema prophylaxis, technical concerns with vessel mismatch, pressure gradients and radiation of anastomoses have led some authors to consider a delayed or distally located LVB (29-31). Pierazzi et al. investigated the outcomes of delayed LVB performed at a location distal to the lymphadenectomy site and after the completion of radiation therapy (29). A total of six patients underwent distal prophylactic LVB between 85 to 130 days after lymphadenectomy with no increased in limb circumference after 12 months of follow-up. Although the number of patients in this study is small, it introduces important considerations of optimal timing and location of distal LVB for lymphedema prophylaxis. In a retrospective review of distal LVB following ALND, Wong et al. reported a 3.8% incidence of lymphedema in the experimental group compared to 17.2% in controls (31). The authors describe benefits of distal LVB to include an improved vessel size match and avoidance of theoretical damage from radiotherapy. Conversely, the bypass of distal lymphatic channels with potentially intact proximal drainage and the implications of delayed LVB thrombosis in prophylactic distal LVB must also be considered. While distal lymphatic reconstruction following lymphadenectomy has been shown to decrease the incidence of lymphedema, refinements to the site, timing, and indication need to be further studied to identify the best outcomes for patients.
Delayed physiologic lymphatic reconstruction
Delayed lymphatic reconstruction is performed to treat established lymphedema. A multidisciplinary care team is critical for these patients, particularly with the involvement of certified lymphedema therapists. Prior to considering lymphatic surgery, patients should have completed CDT, a collection of non-surgical modalities including lymphatic drainage, muscle pumping exercises to promote lymphatic flow, proper skin care, compressive bandaging/garments, and education (32,33). This therapeutic approach, along with a clear assessment of a patient’s baseline measurements, quality of life, and patient compliance to treatment, are important to establish prior to surgical care. Physiologic surgery including LVB, VLNT, and vascularized lymphatic vessel transplant (or transfer) (VLVT) is aimed at treating the fluid burden associated with lymphedema whereas reductive surgery, primarily suction lipectomy, is utilized to reduce the excess fibroadipose tissue. Decision-making between different procedures is complex and involves principally determining the dominant fat to fluid ratio as well as the severity of disease based on symptoms, physical exam and imaging findings.
LVB
LVB involves the anastomosis of lymphatic vessels to nearby venules to distally reroute lymphatic flow into the systemic circulation. LVB is indicated to treat fluid-dominant lymphatic disease, typically in earlier-stage patients. Successful outcomes of LVB are closely linked to the quality of lymphatic channels, namely, the ability to identify functional lymphatic vessels for anastomosis as well as an appropriate recipient venule with significant venous backflow. The MD Anderson (MDA) staging criteria is one of several classification systems based on ICG lymphography patterns (Figure 2) that evaluates the presence of functional linear channels appropriate for LVB in relation to dermal backflow (34). It defines six progressive stages based on the ICG patterns of linear channels in relation to dermal backflow. LVB is typically indicated for MDA stage I or II (34), where functional linear lymphatic channels can be identified on ICG lymphography. In addition to providing diagnostic and staging information, ICG lymphography is commonly employed intraoperatively as it provides real-time mapping of intact linear channels which guide surgical planning. More recently, the utilization of newer adjunctive imaging technologies including magnetic resonance (MR) lymphography and ultra-high frequency ultrasound (UHFUS) have allowed for successful outcomes with LVB in later stages cases as well (35,36).
Multiple anastomotic techniques for LVB have been described, the most common being end-to-end and end-to-side anastomoses (Figure 3) (37). Additional techniques reported in the literature include the side-to-end (38), sleeve-in anastomosis (39,40), “octopus” approach (41), lambda-shaped with intravascular stenting (42,43), pi-shaped (44), modified lambda-shaped anastomosis (43), buffalo-skull (45), and Y-shaped venoplasty (46). These inventive approaches all aim to accomplish maximal drainage of excess lymph fluid and have demonstrated success as independent techniques. However, there is no consensus on the ideal anastomotic approach. AlJindan et al. showed that side-to-end anastomosis conferred greater reduction in limb circumference compared to the end-to-end cohort (38). Other reports have proposed that the quantity of anastomoses correlates to better outcomes (41), while some have contended that better outcomes can be accomplished with LVB in deep lymphatics rather than superficial (47). While multiple techniques can be utilized successfully, the superiority of one over another remains to be determined.
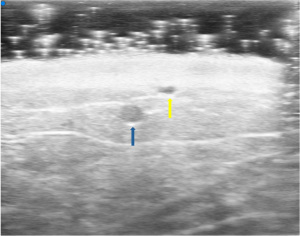
Identification of functional lymphatic vessels as well as nearby venules are a critical component of successfully LVB surgery. UHFUS has emerged as an invaluable preoperative planning tool given its ability to scan at resolutions down to 30 µm (36,48,49). This modality has demonstrated superior visualization of lymph vessel and function compared to conventional high frequency ultrasound (CHFUS) and strong association with lymphatic vessel histology patterns in patients with secondary lymphedema undergoing LVB (36,50). UHFUS (Figure 4) allows for precise localization of lymphatics even in cases of severe dermal backflow (51), selection of larger, ectatic lymphatics and identification of nearby venule branches thereby increasing efficiency in LVB surgery.
Photoacoustic lymphangiography (PAL) has also recently been evaluated as a pre-operative visualization tool for operative planning for LVB (52,53). This technique utilizes a glucose and ICG mixture injected into digit webspaces, followed by near-infrared fluorescent imaging to capture both lymphatic and venous vessel anatomy on a static image. Suzuki et al. found that when compared to mainstay ICG lymphography, the PAL offered better definition of lymphatic vessels, particularly in areas where there is dermal backflow superficial to healthy lymphatic vessels (52).
Venule quality is another critical factor in LVB planning. Venules with backflow can result in venous reflux across the anastomosis and unfavorable pressure gradients increase the risk of anastomotic thrombosis. Visconti et al. investigated the quality of recipient venules and found vessels with increased backflow had poorer postoperative outcomes after LVB (54). Multiple techniques can be utilized to optimize venule selection. Ligating of feeding branches or transcommissural valvuloplasty have been reported (55) as well as other types of venoplasty to minimize LVB backflow (46).
Outcomes after LVB have demonstrated a positive impact both microscopically and clinically in the short and long term. At the cellular level, decreased oxidative stress markers have been reported one month after LVB (56). Patients with a follow-up period of more than three years have demonstrated decreased cellulitis episodes and sustained improvement in quality of life metrics (57). Several studies have shown that compression garment therapy can be reduced or even discontinued after LVB for patients with earlier stage lymphedema (47,58,59). A randomized clinical trial of over 300 cases by Mihara et al. demonstrated that LVB with conservative decongestive therapy reduced lower extremity cellulitis and firmness of tissue compared to non-operative decongestive therapy alone (60). Consensus guidelines based on systematic review by Chang et al. reported that LVB can reduce the severity of lymphedema, particularly for those with early-stage disease (grade 1C) (28).
More recently, robotic-assisted techniques have emerged as a promising advancement in the field of LVB and supermicrosurgery (61-63). In a pilot study by van Mulken et al., anastomosis completion was accomplished in less than half the time with robotic-assisted LVB compared to manual LVB (63). In a 1-year follow-up study, the authors found that those who underwent robot-assisted LVB had slightly increased episodes of postoperative lymphedema maintenance including manual lymphatic drainage and daily compressive garment use (62). Despite this, the two groups were not significantly different in the lymphedema functioning, disability, and health questionnaire that assessed quality of life. As this technology continues to be adopted, additional studies will further define its clinical utility in LVB.
New frontiers have explored the impact of lymphatics on the central nervous system. Impaired meningeal lymphatic flow has been correlated with amyloid beta accumulation, significant in the pathogenesis of dementia and Alzheimer’s disease. Da Mesquita et al. found delivering vascular endothelial growth factor (VEGF)-C, critical to lymphangiogenesis, improved lymphatic function and amyloid beta drainage in murine models (64). Preliminary outcomes following brain lymphatic reconstruction in 50 patients with Alzheimer’s disease demonstrate notable improvements in memory, cognitive function, and behavior after 9-month follow-up; however, this study is ongoing and more objective evidence is needed to support these observations towards the treatment of Alzheimer’s disease (65).
VLNT
VLNTs are another form of physiologic delayed lymphatic reconstruction. VLNTs are typically indicated for patients with fluid-dominant disease. They are utilized in more advanced ICG lymphography patterns without functional linear channels amenable to LVB (66), though simultaneous LVB and VLNT for earlier stage lymphedema has also been advocated given the different physiologic mechanisms of these surgeries (67). Multiple mechanisms have been proposed to explain how VLNT clinically improves lymphedema. Transplanted nodes promote VEGF-C expression, increasing lymphangiogenesis, and creating new lymphatics to further establish intact lymphatic flow (66,68-70). Lymph node flaps can also function as a “pump”, drawing in the fluid from the surrounding tissue and rerouting into the vascular system (69,71). Multiple lymph node donor sites have been described including the omentum, submental, groin, thoracic, supraclavicular and mesenteric sites (72).
The omentum is a common donor site given the minimal risk of donor-site lymphedema (72,73). The omentum has a dual blood supply from the right and left gastroepiploic arteries, permitting a segmental harvest of the omentum. In the context of omental VLNT, generally the entire omentum is not needed, and harvest is usually limited to the right gastroepiploic artery and the associated segment of omentum. Anatomical studies demonstrate and average of 2.6–3.1 number of lymph nodes in this described harvest (73,74), but can be up to 7.3 (75,76). Historically an open laparotomy approach was utilized for omental harvest; however, advances in minimally invasive abdominal surgery and training has increased the use of laparoscopic harvest (77).
Given its native bi-directional venous flow based on the right and left gastroepiploic veins and lack of a capillary bed, a single venous anastomosis can result in venous hypertension of the flap which can ultimately threaten flap viability (78). Different techniques have been described to overcome this venous hypertension including anastomosing both the right gastroepiploic vein and the left gastroepiploic veins in order to restore the native bi-directional venous (76,78,79) as well as additionally creating an arterial flow through flap by anastomosing both right and left gastroepiploic arteries (76). Post-omental VLNT outcomes include lymphedematous extremity circumference reduction ranging from 37.8% to 74.5% (80-83), no recurrence of cellulitis post-operatively (80), and significant improvement in quality of life and patient satisfaction (82-84).
The submental VLNT harvests level Ia and Ib lymph nodes of the neck (66) based on the submental artery (85,86). A platysma-sparing technique aims to minimize the potential negative cosmetic outcome of lower lip motion that can be observed in partial platysma resection as part of the flap (87). Advantages of this flap include the low risk of donor-site lymphedema and an inconspicuous scar of the donor site. The variability of number of lymph nodes in the submental region may warrant preoperative imaging to select the side with the higher number of lymph nodes within the submental flap as some studies suggest the number of lymph nodes transferred may correlate with limb circumference reduction (88,89).
The groin offers a rich superficial lymphatic basin that drains the abdomen and flanks based on the superficial circumflex iliac vessels along with a concealed donor site that makes it a favorable VLNT option (90). Of note, the deep lymphatics of the groin drain the lower extremity, typically residing below the groin crease, and must be avoided. Reverse lymphatic mapping is now routinely employed to minimize the risk of iatrogenic lymphedema. This technique involves the injection of radioisotope in the ipsilateral lower extremity of the groin VLNT as well as ICG in the abdomen/flanks, allowing the surgeon to avoid the sentinel nodes draining the leg while identifying the desired superficial nodes for the flap (Figure 5) (91). More recently, single-photon emission computed tomography-computed tomography (SPECT-CT) has been described for preoperative reverse mapping to identify aberrant drainage of the lower extremity into the superficial lymphatic system (92). Groin VLNT in conjunction with physiotherapy and compression was found to have a 57% limb volume reduction compared to the non-surgical cohort treated with physiotherapy and compression alone who experienced 18% limb volume reduction in one randomized control study (93). A prospective study found similar results with a 40.4% circumferential reduction rate in the groin VLNT group compared to 8.3% in the physical therapy alone group after an average follow-up period of 3.3 years (94).
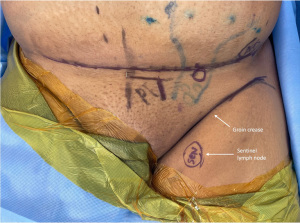
The superficial circumflex iliac artery perforator (SCIP) flap can be combined with the associated groin lymph nodes, otherwise known as a composite SCIP (69,95). In cases where patients are at risk of developing post-oncologic or post-traumatic lymphedema along with a soft tissue defect, a prophylactic VLNT may also be performed at the time of soft tissue coverage (96,97). The lymphatic system transfer (LYST) builds upon the composite SCIP VLNT to include afferent lymph vessels as a means of potentially reducing the time needed for lymphangiogenesis (98).
The combination of a groin VLNT and an abdominal-based autologous flap can also be used for breast reconstruction and for treatment of lymphedema simultaneously (99-101). Multiple studies have demonstrated improved lymphedema-related quality of life for patients following this combined reconstruction (102,103). While it is possible to harvest both flaps as a unit (104), they may also be harvested separately with possible orthotopic inset of the VLNT more distally on the affected extremity (105).
The lateral thoracic VLNT utilizes the level 1 axillary lymph nodes with a pedicle from the lateral thoracic artery or the thoracodorsal artery (69,71,106). As with groin lymph nodes, harvesting the thoracic lymph nodes poses a risk of donor site lymphedema, and reverse lymphatic mapping is critical (91). A benefit of this flap includes a long vascular pedicle, the ability to include a skin paddle and a relatively high number of lymph nodes within the transfer (106), though anatomic variability in perfusion often requires isolating both the thoracodorsal and lateral thoracic system, with a potential need to anastomose both pedicles. The thoracic VLNT may also be harvested in a pedicled fashion with a partial latissimus flap for ipsilateral lymphedema after ALND, also providing soft tissue bulk if needed for the axilla (107).
The supraclavicular lymph node flap is comprised of the level Vb nodes of the neck and is supplied by the transverse cervical artery (108,109). It offers straightforward anatomical landmarks with low risk of donor site lymphedema. However, care must be taken to avoid injury to surrounding critical structures including the carotid arteries, internal jugular vein, phrenic nerve, and right lymphatic and left thoracic ducts (108). Safety has been demonstrated in large series, included a prospective trial of 100 cases, with two reported infections and three cases of chyle leak (110). In this series, the chyle leak was treated conservatively with medium chain fatty acid diet and drainage; however, high volume chyle leak following supraclavicular VLNT can be significantly more difficult to manage (111).
Recipient site location for VLNT is also an important consideration. Orthotopic transplants are placed anatomically and proximally such as in the axilla or groin, whereas heterotopic VLNTs are transplanted non-anatomical and distally such as near the ankle or wrist (66,108). The advantages of an orthotopic recipient site include cosmetic scar location and restoring lymph nodes at the site of removal, though fibrosis effects from previous surgery or radiation can increase difficulty. Heterotopic VLNTs have more visible scars and can be bulky depending on the flap choice, though gravitational forces and pathophysiologic retrograde lymphatic flow may shift impaired lymph movement towards the flap (66,69,94,112).
Although the exact mechanism of VLNT continues to be elucidated, improved outcomes for patients are widely reported. VLNT has a high level of evidence (grade 1B) as an effective surgical treatment for reducing the severity of lymphedema (28). A recent meta-analysis identified 31 studies (581 patients) and reviewed outcomes in limb volume, cellulitis episodes, and quality of life scores, all of which improved following VLNT (113). A prospective study by Brown et al. demonstrated significant improvement of average limb reduction and reduction in cellulitis after two years in 89 patients who underwent either omentum, lateral thoracic, supraclavicular or groin VLNT, with 34% of patients no longer requiring compression therapy (79). VLNT have been demonstrated to be more effective in reducing limb circumference and incidence of cellulitis compared to compression and decongestive therapies alone (28).
Combined lymphatic surgery approaches
The multiple presentations of lymphedema with regards to fluid and fat ratios as well as severity have led to the utilization of combined surgical approaches to address different components of the disease. This can include combination physiologic and reductive procedures as well as different physiologic procedures at one time. In a cohort of 21 patients, Brazio et al. demonstrated that LVB or VLNT with simultaneous liposuction confers benefits related to decreased lymphedematous volume and episodes of cellulitis, and a meaningful reduction in the duration of post-operative compression garment application (114). A single stage combination procedure may be prudent when there is both fluid and fibroadipose presentation and it is unclear which is the dominating pathology, which has been consistent with other reports that have additionally shown patient reports of improved clinical symptoms (115) and quality of life (116).
Combined physiologic approaches have demonstrated success in a diverse group inclusive of early and late-stage lymphedema, primary and secondary etiologies, and lymphedema localized to different anatomic regions (67,117-120). Chang et al. was able to demonstrate a volume reduction rate of 20–36% and improved quality of life scores over 4 years post-operatively in patients with primary, early-stage secondary, and late-stage secondary lymphedema who underwent combined LVB and VLNT procedures (67). As LVB and VLNT have different mechanisms to treat fluid burden, these procedures may be flexibly utilized for patients regardless of their staging. Institutions have proposed different algorithms to determine which patients may be suitable candidates for a combined approach (114,117,118), and while there is no consensus, it is increasingly evident that this mixed approach may yield improved results than standalone procedures for specific patients.
VLVT
VLVT is a technique that isolates lymphatic vessels within a soft tissue flap that is transferred to an affected area without lymphatic vessel anastomosis or node transplant (121-125). Mechanisms of action are attributed to lymphangiogenesis conferred by the perfused lymphatics within the flap (124). The technique has been advocated for patients who wish to avoid donor site lymphedema (121) and can be combined with VLNT other lymphatic reconstruction techniques to optimize patient outcomes (122).
Chen et al. demonstrated improvement in lymphedema symptoms in six patients who underwent SCIP-based VLVT for fluid dominant lymphedema (124). The superficial lymphatics within the region of the SCIP flap were identified using ICG lymphography, and the flap was raised in a non-anatomic superficial plane to capture lymphatic vessels but spare the superficial inguinal lymph nodes. At 1-year follow-up, all six patients showed decreased limb circumference measurements, and the emergence of new linear patterns on post-operative lymphography. While early results are promising, larger studies with longer follow-up are needed to further elucidate efficacy and indications.
While VLVT is described mainly for the treatment of chronic lymphedema, it may have a role in the prophylactic management of acute extremity wounds. Yamamoto et al. investigated the importance of aligning the axis of lymphatics within a flap to the axis of the extremity requiring soft tissue coverage (126). In coverage of acute traumatic wounds or oncologic defects of the extremities, lymph-interpositional-flap transfer or LIFT, which used lymphography to help design and guide inset of the flap to align the axis of lymphatic flow within the flap parallel to the axis of the affected extremity, had lower rates of lymphedema and a higher rate of restored lymphatic flow compared to traditional soft tissue flaps. Additionally, a subset of LIFT flaps where the lymphatic stumps in the flap were approximated within 2 cm to the lymphatic stumps of the recipient site (LIFT+) had higher rates of restored lymphatic flow and lower rates of lymphedema compared to the group where the lymphatics stumps were farther than 2 cm apart (LIFT−). This study indicates the importance of considering lymphatic directionality of a flap during VLVT as well as possible distance limitations of lymphangiogenesis.
Reductive surgery
Suction-assisted lipectomy (SAL)
The progression of lymphedema results in the transition from a fluid to a fibroadipose dominant state. In patients with more advanced disease, reductive procedures including both SAL as well as excisional techniques are effective means of removing this excess fibroadipose tissue. SAL involves liposuction of affected lymphedematous extremities to remove excess adipose deposition and is indicated for patients with fat-dominant or mixed fluid and fatty disease. These procedures are typically performed using tourniquet control and tumescent infiltration to minimize blood loss.
SAL has shown up to 94% reduction in lower extremity volume, decreased cellulitis recurrence, enhanced range of motion, and improved quality of life. For cases of upper extremity lymphedema, Brorson et al. showed up to 104% reduction amongst patients treated with SAL and controlled compression versus those on compression alone (127). Meta-analysis of 6 case studies (294 patients) showed an average limb volume reduction of 1,702 mL in those treated with liposuction combined with compression therapy (28). SAL has furthermore been shown to improve lymphedema symptoms (128), patient quality-of-life based on validated questionnaires (129), and long-term volume reduction up to over 20 years in appropriately managed patients (9). Consensus guidelines conclude there is a role for SAL in the treatment of moderate to severe lymphedema as it targets the non-fluid component of the advanced disease; however, timing and staging with physiologic procedures have yet to be determined (grade 1C) (28).
Compression garments are a critical component of postoperative management after SAL to minimize fluid accumulation in the affected extremity. While case reports have suggested that liposuction may actually improve lymph fluid transport based on postoperative lymphoscintigraphy (10), these procedures remain targeted at addressing the fibroadipose and not fluid component of lymphedema. In patients with mixed disease, staged approaches have been recommended combining both physiologic and reductive procedures to address these different components (130).
Excisional reductive techniques
Excisional reductive techniques can be considered for volume reduction and symptomatic relief in chronic severe lymphedema where there is extensive tissue fibrosis and hypertrophy. One of the oldest approaches to debulking advanced cases of lymphedema, the Charles procedure, excises tissue to the level of fascia and covers the wounds with skin grafts. The Charles procedure is effective in reducing limb size and removing lymphedematous tissue but can carry significant complication risks including infection, significant blood loss requiring transfusion, skin graft failure, chronic wounds and even amputation (131-134).
The invasive nature of the Charles procedure has led to the evolution of modified approaches. Staging debulking the debulking and grafting procedures aims to offset the physiologic burden placed on the body (8). The Homans procedure is another variation that utilizes longitudinal incisions along the extremity, subdermal debulking and preservation of the skin (135-137). These excisional approaches have found relevance in modern lymphedema treatment as part of an integrated approach of excision and physiologic restoration for cases of severe or end stage lymphedema. Ciudad et al. describe a combined, single-stage protocol of the Charles procedure, Homan’s procedures, and VLNT on 68 patients with stage III lower extremity lymphedema with no major complications (138). Sapountzis et al. modified the Charles procedure to spare the superficial venous system and lesser saphenous vein as outflow options for VLNT (139). A similar, combined excisional and VLNT approach in 29 patients with bilateral lower extremity demonstrated a significant decrease in cellulitis and increase in quality of life (133).
Additional considerations
Insurance coverage
Despite the burden of lymphedema on over a million Americans, legislation and policy to mandate insurance coverage for lymphedema therapy has been slow to become reality and many limitations remain. Conservative therapeutic regimens such as compression garments, manual lymphatic drainage, and physical therapy have historically been covered on a case-by-case basis with wide variance based on a patient’s insurance plan or state (140,141). Given the routine cadence needed to manage lymphedema including regular garment changes and therapeutic appointments, costs can easily exceed 3,000 USD annually (142). Tenuous coverage for conservative measures may not only impose a cost burden on patients but can also influence treatment compliance and ultimately lead to increased medical costs, hospitalizations, and complications. While the spectrum of nonoperative lymphedema therapies has yet to be fully covered, the Lymphedema Treatment Act (LTA), effective 2024, reflects a monumental stride forward (143). This legislation mandates insurance companies to cover medically necessary compression garments for lymphedema treatment, affording access to a mainstay of decongestive therapy. Notably, the LTA is actualized over a decade after its initial conception, signaling that the expansion of medically necessary treatments for lymphedema requires vigilance for success.
Despite the safety profile and successful outcomes of lymphedema surgery, countless obstacles have been faced by providers and patients alike as most insurance companies do not formally acknowledge surgical intervention for lymphedema as standard of care (144-146). ILR remains an elective procedure not covered by more than 50% of insurance companies and LVB and VLNT are also commonly denied by insurance providers (145,146). Part of this dilemma may be due to the variable coding taxonomy which lends to inconsistent pricing rates for surgical treatment not only between physiologic and reductive procedures, but within each type as well (147). While federal policy has yet to materialize, a major accomplishment involves the collaboration between the Boston Lymphatic Center, Lymphatic Education and Research Network, and Blue Cross Blue Shield Massachusetts (148). This work highlighted the disconnect that can exist amongst disciplines and reinforced the necessity of multidisciplinary efforts to bridge knowledge gaps pertaining to evidence-based practices, fiscal plans, and operative outcomes for lymphedema. Together, a policy was formulated for lymphatic procedures by standardization of the lexicon and measurements involved in the approach for evaluation, diagnostic workup, treatment, and outcomes.
Conclusions
Lymphedema is a burdensome disease for patients that impacts daily quality of life and carries significant morbidity in advanced stages. The advent of surgical treatment modalities for lymphedema and recent advancements in supermicrosurgery and imaging technologies offer patients and providers an expanded toolkit to treat lymphedema. This review demonstrates the utility of modern physiologic and reductive surgical techniques as both treatment and preventative tools. Additionally, these approaches do not have to exist in silos; rather, techniques are versatile and should be combined as well as individualized to fit each patient’s specific needs. As surgical treatment options continue to demonstrate their utility, it is important for policy and insurance to align in coding nomenclature and fiscal rates to ensure equitable access.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-49/rc
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-49/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-49/coif). A.A.S. serves as an unpaid editorial board member of Translational Breast Cancer Research from December 2023 to November 2025. A.A.S. is a research consultant for Abbvie Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Azhar SH, Lim HY, Tan BK, et al. The Unresolved Pathophysiology of Lymphedema. Front Physiol 2020;11:137. [Crossref] [PubMed]
- Tashiro K, Feng J, Wu SH, et al. Pathological changes of adipose tissue in secondary lymphoedema. Br J Dermatol 2017;177:158-67. [Crossref] [PubMed]
- Sleigh BC, Manna B. Lymphedema. StatPearls. Treasure Island (FL): StatPearls Publishing; 2018.
- Campisi CC, Boccardo F, Ryan M, et al. The campisi approach for lymphatic surgery. In: Cheng MH, Chang DW, Patel KM. Principles and Practice of Lymphedema Surgery (Second Edition). Elsevier; 2022:165-73.
- Greene AK, Zurakowski D, Goss JA. Body Mass Index and Lymphedema Morbidity: Comparison of Obese versus Normal-Weight Patients. Plast Reconstr Surg 2020;146:402-7. [Crossref] [PubMed]
- Sleigh BC, Manna B. Lymphedema. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
- Scaglioni MF, Arvanitakis M, Chen YC, et al. Comprehensive review of vascularized lymph node transfers for lymphedema: Outcomes and complications. Microsurgery 2018;38:222-9. [Crossref] [PubMed]
- Hague A, Bragg T, Thomas M, et al. Severe lower limb lymphoedema successfully treated with a two-stage debulking procedure: a case report. Case Reports Plast Surg Hand Surg 2020;7:38-42. [Crossref] [PubMed]
- Brorson H. Complete Reduction of Arm Lymphedema Following Breast Cancer – A Prospective Twenty-One Years’ Study. Plastic and Reconstructive Surgery 2015;136:134-5. [Crossref]
- Greene AK, Voss SD, Maclellan RA. Liposuction for Swelling in Patients with Lymphedema. N Engl J Med 2017;377:1788-9. [Crossref] [PubMed]
- Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery 2014;34:421-4. [Crossref] [PubMed]
- Feldman S, Bansil H, Ascherman J, et al. Single Institution Experience with Lymphatic Microsurgical Preventive Healing Approach (LYMPHA) for the Primary Prevention of Lymphedema. Ann Surg Oncol 2015;22:3296-301. [Crossref] [PubMed]
- Hahamoff M, Gupta N, Munoz D, et al. A Lymphedema Surveillance Program for Breast Cancer Patients Reveals the Promise of Surgical Prevention. J Surg Res 2019;244:604-11. [Crossref] [PubMed]
- Johnson AR, Singhal D. Immediate lymphatic reconstruction. J Surg Oncol 2018;118:750-7. [Crossref] [PubMed]
- Johnson AR, Kimball S, Epstein S, et al. Lymphedema Incidence After Axillary Lymph Node Dissection: Quantifying the Impact of Radiation and the Lymphatic Microsurgical Preventive Healing Approach. Ann Plast Surg 2019;82:S234-41. [Crossref] [PubMed]
- Coriddi M, Dayan J, Bloomfield E, et al. Efficacy of Immediate Lymphatic Reconstruction to Decrease Incidence of Breast Cancer-related Lymphedema: Preliminary Results of Randomized Controlled Trial. Ann Surg 2023;278:630-7. [Crossref] [PubMed]
- Friedman R, Bustos VP, Postian T, et al. Utilizing a lower extremity vein graft for immediate lymphatic reconstruction. J Plast Reconstr Aesthet Surg 2022;75:2831-70. [Crossref] [PubMed]
- Brahma B, Yamamoto T, Panigoro SS, et al. Supermicrosurgery lymphaticovenous and lymphaticolymphatic anastomosis: Technical detail and short-term follow-up for immediate lymphatic reconstruction in breast cancer treatment-related lymphedema prevention. J Vasc Surg Venous Lymphat Disord 2024;12:101863. [Crossref] [PubMed]
- Spoer DL, Berger LE, Towfighi PN, et al. Lymphovenous Coupler-Assisted Bypass for Immediate Lymphatic Reconstruction. J Reconstr Microsurg 2024;40:334-47. [Crossref] [PubMed]
- Allan AY, Mughal M, Mohanna PN, et al. Lymphovenous anastomosis using the venous coupler: Primary prevention of lymphoedema. J Plast Reconstr Aesthet Surg 2024;92:282-4. [Crossref] [PubMed]
- Brown S, Kokosis G, Graziano FD, et al. Immediate Lymphatic Reconstruction with Vascularized Omentum Lymph Node Transplant: Reducing the Risk of Both Painful Contracture and Lymphedema. Plast Reconstr Surg Glob Open 2024;12:e5747. [Crossref] [PubMed]
- Spiguel L, Shaw C, Katz A, et al. Fluorescein Isothiocyanate: A Novel Application for Lymphatic Surgery. Ann Plast Surg 2017;78:S296-8. [Crossref] [PubMed]
- Alarcón PZ, Torrano L, Ibarra A, et al. Prophylactic lymphedema surgery in lower limb soft tissue sarcomas: A clinical paradigm in a promising field. J Plast Reconstr Aesthet Surg 2024;88:524-34. [Crossref] [PubMed]
- Cakmakoglu C, Kwiecien GJ, Schwarz GS, et al. Lymphaticovenous Bypass for Immediate Lymphatic Reconstruction in Locoregional Advanced Melanoma Patients. J Reconstr Microsurg 2020;36:247-52. [Crossref] [PubMed]
- Takeishi M, Kojima M, Mori K, et al. Primary intrapelvic lymphaticovenular anastomosis following lymph node dissection. Ann Plast Surg 2006;57:300-4. [Crossref] [PubMed]
- Morotti M, Menada MV, Boccardo F, et al. Lymphedema microsurgical preventive healing approach for primary prevention of lower limb lymphedema after inguinofemoral lymphadenectomy for vulvar cancer. Int J Gynecol Cancer 2013;23:769-74. [Crossref] [PubMed]
- Han S, Mast B. Safety and Efficacy of Immediate Lymphatic Reconstruction in Patients with Melanoma: A Systematic Review. Plast Reconstr Surg Glob Open 2023;11:96. [Crossref]
- Chang DW, Dayan J, Greene AK, et al. Surgical Treatment of Lymphedema: A Systematic Review and Meta-Analysis of Controlled Trials. Results of a Consensus Conference. Plast Reconstr Surg 2021;147:975-93. [Crossref] [PubMed]
- Pierazzi DM, Arleo S, Faini G. Distally Prophylactic Lymphaticovenular Anastomoses after Axillary or Inguinal Complete Lymph Node Dissection Followed by Radiotherapy: A Case Series. Medicina (Kaunas) 2022;58:207. [Crossref] [PubMed]
- Chen WF, Knackstedt R. Delayed Distally Based Prophylactic Lymphaticovenular Anastomosis: Improved Functionality, Feasibility, and Oncologic Safety? J Reconstr Microsurg 2020;36:e1-2. [Crossref] [PubMed]
- Wong AW, Sim NHS, Thing CB, et al. Distally Based Lymphatic Microsurgical Preventive Healing Approach-A Modification of the Classic Approach. Arch Plast Surg 2024;51:504-9. [Crossref] [PubMed]
- Chang DW, Masia J, Garza R 3rd, et al. Lymphedema: Surgical and Medical Therapy. Plast Reconstr Surg 2016;138:209S-218S. [Crossref] [PubMed]
- The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020;53:3-19. [PubMed]
- Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 2013;132:1305-14. [Crossref] [PubMed]
- Cha HG, Oh TM, Cho MJ, et al. Changing the Paradigm: Lymphovenous Anastomosis in Advanced Stage Lower Extremity Lymphedema. Plast Reconstr Surg 2021;147:199-207. [Crossref] [PubMed]
- Hayashi A, Giacalone G, Yamamoto T, et al. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast Reconstr Surg Glob Open 2019;7:e2086. [Crossref] [PubMed]
- Kwon JG, Jeong S, Pak CJ, et al. Comparative Analysis between Side-to-End and End-to-End Lymphaticovenous Anastomosis for Secondary Lower Limb Lymphedema. Plast Reconstr Surg 2022;150:1138-48. [PubMed]
- AlJindan FK, Lin CY, Cheng MH. Comparison of Outcomes between Side-to-End and End-to-End Lymphovenous Anastomoses for Early-Grade Extremity Lymphedema. Plast Reconstr Surg 2019;144:486-96. [Crossref] [PubMed]
- Chung JH, Baek SO, Park HJ, et al. Efficacy and patient satisfaction regarding lymphovenous bypass with sleeve-in anastomosis for extremity lymphedema. Arch Plast Surg 2019;46:46-56. [Crossref] [PubMed]
- Yamamoto Y, Sugihara T. Microsurgical lymphaticovenous implantation for the treatment of chronic lymphedema. Plast Reconstr Surg 1998;101:157-61. [Crossref] [PubMed]
- Chen WF, Yamamoto T, Fisher M, et al. The "Octopus" Lymphaticovenular Anastomosis: Evolving Beyond the Standard Supermicrosurgical Technique. J Reconstr Microsurg 2015;31:450-7. [Crossref] [PubMed]
- Narushima M, Mihara M, Yamamoto Y, et al. The intravascular stenting method for treatment of extremity lymphedema with multiconfiguration lymphaticovenous anastomoses. Plast Reconstr Surg 2010;125:935-43. [Crossref] [PubMed]
- Yamamoto T, Yoshimatsu H, Yamamoto N, et al. Modified lambda-shaped lymphaticovenular anastomosis with supermicrosurgical lymphoplasty technique for a cancer-related lymphedema patient. Microsurgery 2014;34:308-10. [Crossref] [PubMed]
- Ayestaray B, Bekara F. π-shaped lymphaticovenular anastomosis: the venous flow sparing technique for the treatment of peripheral lymphedema. J Reconstr Microsurg 2014;30:551-60. [Crossref] [PubMed]
- Brahma B, Yamamoto T, Agdelina C, et al. Immediate-delayed lymphatic reconstruction after axillary lymph nodes dissection for locally advanced breast cancer-related lymphedema prevention: Report of two cases. Microsurgery 2024;44:e31033. [Crossref] [PubMed]
- Akita S, Yamaji Y, Tokumoto H, et al. Prevention of venous reflux with full utilization of venoplasty in lymphaticovenular anastomosis. J Plast Reconstr Aesthet Surg 2020;73:537-43. [Crossref] [PubMed]
- Scaglioni MF, Meroni M, Fritsche E. Combining superficial and deep lymphovenous anastomosis for lymphedema treatment: Preliminary results. Microsurgery 2022;42:22-31. [Crossref] [PubMed]
- Izzetti R, Vitali S, Aringhieri G, et al. Ultra-High Frequency Ultrasound, A Promising Diagnostic Technique: Review of the Literature and Single-Center Experience. Can Assoc Radiol J 2021;72:418-31. [Crossref] [PubMed]
- Hayashi A, Visconti G, Yamamoto T, et al. Intraoperative imaging of lymphatic vessel using ultra high-frequency ultrasound. J Plast Reconstr Aesthet Surg 2018;71:778-80. [Crossref] [PubMed]
- Bianchi A, Visconti G, Hayashi A, et al. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: A step forward in lymphatic surgery. J Plast Reconstr Aesthet Surg 2020;73:1622-9. [Crossref] [PubMed]
- Visconti G, Hayashi A, Bianchi A, et al. Lymphaticovenular Anastomosis for Advanced-Stage Peripheral Lymphedema: Expanding Indication and Introducing the Hand/Foot Sign. J Plast Reconstr Aesthet Surg 2022;75:2153-63. [Crossref] [PubMed]
- Suzuki Y, Kajita H, Oh A, et al. Photoacoustic lymphangiography exhibits advantages over near-infrared fluorescence lymphangiography as a diagnostic tool in patients with lymphedema. J Vasc Surg Venous Lymphat Disord 2022;10:454-462.e1. [Crossref] [PubMed]
- Suzuki Y, Kajita H, Watanabe S, et al. Surgical Applications of Lymphatic Vessel Visualization Using Photoacoustic Imaging and Augmented Reality. J Clin Med 2021;11:194. [Crossref] [PubMed]
- Visconti G, Salgarello M, Hayashi A. The Recipient Venule in Supermicrosurgical Lymphaticovenular Anastomosis: Flow Dynamic Classification and Correlation with Surgical Outcomes. J Reconstr Microsurg 2018;34:581-9. [Crossref] [PubMed]
- Akita S, Mitsukawa N, Kuriyama M, et al. External valvuloplasty for subcutaneous small veins to prevent venous reflux in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg 2013;132:1008-14. [Crossref] [PubMed]
- Yang JC, Huang LH, Wu SC, et al. Lymphaticovenous Anastomosis Supermicrosurgery Decreases Oxidative Stress and Increases Antioxidant Capacity in the Serum of Lymphedema Patients. J Clin Med 2021;10:1540. [Crossref] [PubMed]
- Cheng MH, Tee R, Chen C, et al. Simultaneous Ipsilateral Vascularized Lymph Node Transplantation and Contralateral Lymphovenous Anastomosis in Bilateral Extremity Lymphedema with Different Severities. Ann Surg Oncol 2020;27:5267-76. [Crossref] [PubMed]
- Jonis YMJ, Wolfs JAGN, Hummelink S, et al. The 6 month interim analysis of a randomized controlled trial assessing the quality of life in patients with breast cancer related lymphedema undergoing lymphaticovenous anastomosis vs. conservative therapy. Sci Rep 2024;14:2238. [Crossref] [PubMed]
- Qiu SS, Pruimboom T, Cornelissen AJM, et al. Outcomes following lymphaticovenous anastomosis (LVA) for 100 cases of lymphedema: results over 24-months follow-up. Breast Cancer Res Treat 2020;184:173-83. [Crossref] [PubMed]
- Mihara M, Hara H, Kawasaki Y, et al. Lymphatic venous anastomosis and complex decongestive therapy for lymphoedema: randomized clinical trial. Br J Surg 2024;111:znad372. [Crossref] [PubMed]
- Weinzierl A, Barbon C, Gousopoulos E, et al. Benefits of robotic-assisted lymphatic microsurgery in deep anatomical planes. JPRAS Open 2023;37:145-54. [Crossref] [PubMed]
- van Mulken TJM, Wolfs JAGN, Qiu SS, et al. One-Year Outcomes of the First Human Trial on Robot-Assisted Lymphaticovenous Anastomosis for Breast Cancer-Related Lymphedema. Plast Reconstr Surg 2022;149:151-61. [Crossref] [PubMed]
- van Mulken TJM, Schols RM, Scharmga AMJ, et al. First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: a randomized pilot trial. Nat Commun 2020;11:757. [Crossref] [PubMed]
- Da Mesquita S, Papadopoulos Z, Dykstra T, et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 2021;593:255-60. [Crossref] [PubMed]
- Xie Q, Louveau A, Pandey S, et al. Rewiring the Brain: The Next Frontier in Supermicrosurgery. Plast Reconstr Surg 2024;153:494e-495e. [PubMed]
- Schaverien MV, Coroneos CJ. Surgical Treatment of Lymphedema. Plast Reconstr Surg 2019;144:738-58. [Crossref] [PubMed]
- Garza RM, Beederman M, Chang DW. Physical and Functional Outcomes of Simultaneous Vascularized Lymph Node Transplant and Lymphovenous Bypass in the Treatment of Lymphedema. Plast Reconstr Surg 2022;150:169-80. [Crossref] [PubMed]
- Garza RM, Wong D, Chang DW. Optimizing Outcomes in Lymphedema Reconstruction. Plast Reconstr Surg 2023;152:1131e-42e. [Crossref] [PubMed]
- Pappalardo M, Patel K, Cheng MH. Vascularized lymph node transfer for treatment of extremity lymphedema: An overview of current controversies regarding donor sites, recipient sites and outcomes. J Surg Oncol 2018;117:1420-31. [Crossref] [PubMed]
- Aschen SZ, Farias-Eisner G, Cuzzone DA, et al. Lymph node transplantation results in spontaneous lymphatic reconnection and restoration of lymphatic flow. Plast Reconstr Surg 2014;133:301-10. [Crossref] [PubMed]
- Gould DJ, Mehrara BJ, Neligan P, et al. Lymph node transplantation for the treatment of lymphedema. J Surg Oncol 2018;118:736-42. [Crossref] [PubMed]
- Jarvis NR, Torres RA, Avila FR, et al. Vascularized omental lymphatic transplant for upper extremity lymphedema: A systematic review. Cancer Rep (Hoboken) 2021;4:e1370. [Crossref] [PubMed]
- Howell AC, Gould DJ, Mayfield C, et al. Anatomical Basis of the Gastroepiploic Vascularized Lymph Node Transfer: A Radiographic Evaluation Using Computed Tomographic Angiography. Plast Reconstr Surg 2018;142:1046-52. [Crossref] [PubMed]
- Cook JA, Sasor SE, Tholpady SS, et al. Omental Vascularized Lymph Node Flap: A Radiographic Analysis. J Reconstr Microsurg 2018;34:472-7. [Crossref] [PubMed]
- Yoo H, Hong KY, Min S, et al. Distribution of Perigastric Station 4d Lymph Nodes in Vascularized Gastroepiploic Lymph Node Transfer: An Anatomic Study and Case Series. Ann Surg Oncol 2024;31:3694-704. [Crossref] [PubMed]
- Johnson AR, Bravo MG, Granoff MD, et al. Flow-through Omental Flap for Vascularized Lymph Node Transfer: A Novel Surgical Approach for Delayed Lymphatic Reconstruction. Plast Reconstr Surg Glob Open 2019;7:e2436. [Crossref] [PubMed]
- Nguyen AT, Suami H, Hanasono MM, et al. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J Surg Oncol 2017;115:84-9. [Crossref] [PubMed]
- Dayan JH, Voineskos S, Verma R, et al. Managing Venous Hypertension in Vascularized Omentum Lymphatic Transplant: Restoring Bidirectional Venous Drainage. Plast Reconstr Surg 2018;141:326e-327e. [Crossref] [PubMed]
- Brown S, Mehrara BJ, Coriddi M, et al. A Prospective Study on the Safety and Efficacy of Vascularized Lymph Node Transplant. Ann Surg 2022;276:635-53. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Date S, et al. Double gastroepiploic vascularized lymph node tranfers to middle and distal limb for the treatment of lymphedema. Microsurgery 2017;37:771-9. [Crossref] [PubMed]
- Agko M, Ciudad P, Chen HC. Staged surgical treatment of extremity lymphedema with dual gastroepiploic vascularized lymph node transfers followed by suction-assisted lipectomy-A prospective study. J Surg Oncol 2018;117:1148-56. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Adabi K, et al. Combined double vascularized lymph node transfers and modified radical reduction with preservation of perforators for advanced stages of lymphedema. J Surg Oncol 2019;119:439-48. [Crossref] [PubMed]
- Ciudad P, Maruccia M, Socas J, et al. The laparoscopic right gastroepiploic lymph node flap transfer for upper and lower limb lymphedema: Technique and outcomes. Microsurgery 2017;37:197-205. [Crossref] [PubMed]
- Manrique OJ, Bustos SS, Kapoor T, et al. Gastroepiploic vascularized lymph node transfer for the treatment of extremity lymphedema: comparison between middle and distal inset. Gland Surg 2020;9:528-38. [Crossref] [PubMed]
- Cheng MH, Huang JJ, Nguyen DH, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol 2012;126:93-8. [Crossref] [PubMed]
- Tzou CH, Meng S, Ines T, et al. Surgical anatomy of the vascularized submental lymph node flap: Anatomic study of correlation of submental artery perforators and quantity of submental lymph node. J Surg Oncol 2017;115:54-9. [Crossref] [PubMed]
- Poccia I, Lin CY, Cheng MH. Platysma-sparing vascularized submental lymph node flap transfer for extremity lymphedema. J Surg Oncol 2017;115:48-53. [Crossref] [PubMed]
- Paulus VAA, Winters H, Hummelink S, et al. Submental flap for vascularized lymph node transfer; a CTA-based study on lymph node distribution. J Surg Oncol 2020;122:1226-31. [Crossref] [PubMed]
- Gustafsson J, Chu SY, Chan WH, et al. Correlation between Quantity of Transferred Lymph Nodes and Outcome in Vascularized Submental Lymph Node Flap Transfer for Lower Limb Lymphedema. Plast Reconstr Surg 2018;142:1056-63. [Crossref] [PubMed]
- Tourani SS, Taylor GI, Ashton MW. Vascularized Lymph Node Transfer: A Review of the Current Evidence. Plast Reconstr Surg 2016;137:985-93. [Crossref] [PubMed]
- Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: a new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277-85. [Crossref] [PubMed]
- Broyles JM, Smith JM, Wong FC, et al. Single-Photon Emission Computed Tomographic Reverse Lymphatic Mapping for Groin Vascularized Lymph Node Transplant Planning. Plast Reconstr Surg 2022;150:869e-879e. [PubMed]
- Dionyssiou D, Demiri E, Tsimponis A, et al. A randomized control study of treating secondary stage II breast cancer-related lymphoedema with free lymph node transfer. Breast Cancer Res Treat 2016;156:73-9. [Crossref] [PubMed]
- Cheng MH, Chen SC, Henry SL, et al. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286-98. [Crossref] [PubMed]
- Akita S, Mitsukawa N, Kubota Y, et al. Delayed Partial Breast Reconstruction and Vascularized Lymph Node Transfer by a Superficial Circumflex Iliac Artery Perforator Flap. Plast Reconstr Surg 2016;137:490e-491e. [Crossref] [PubMed]
- Yamamoto T, Saito T, Ishiura R, et al. Quadruple-component superficial circumflex iliac artery perforator (SCIP) flap: A chimeric SCIP flap for complex ankle reconstruction of an exposed artificial joint after total ankle arthroplasty. J Plast Reconstr Aesthet Surg 2016;69:1260-5. [Crossref] [PubMed]
- Scaglioni MF, Meroni M, Fritsche E, et al. The use of pedicled chimeric superficial circumflex iliac artery perforator (SCIP) flap as lymphatic interpositional flap for deep thigh defect reconstruction: A case report. Microsurgery 2022;42:360-5. [Crossref] [PubMed]
- Yoshimatsu H, Visconti G, Karakawa R, et al. Lymphatic System Transfer for Lymphedema Treatment: Transferring the Lymph Nodes with Their Lymphatic Vessels. Plast Reconstr Surg Glob Open 2020;8:e2721. [Crossref] [PubMed]
- Nguyen AT, Chang EI, Suami H, et al. An algorithmic approach to simultaneous vascularized lymph node transfer with microvascular breast reconstruction. Ann Surg Oncol 2015;22:2919-24. [Crossref] [PubMed]
- Wu CH, Velazquez-Mujica J, Donfrancesco A, et al. Classification of the Conjoined Latissimus Dorsi-Groin Flap and Indications for the Four Types of the Flap. Plast Reconstr Surg 2024;153:632e-635e. [PubMed]
- Chen R, Mu L, Zhang H, et al. Simultaneous breast reconstruction and treatment of breast cancer-related upper arm lymphedema with lymphatic lower abdominal flap. Ann Plast Surg 2014;73:S12-7. [Crossref] [PubMed]
- Winters H, Tielemans HJP, Hummelink S, et al. DIEP flap breast reconstruction combined with vascularized lymph node transfer for patients with breast cancer-related lymphedema. Eur J Surg Oncol 2022;48:1718-22. [Crossref] [PubMed]
- Di Taranto G, Coleman GJ, Hardwicke J, et al. A comparative study between deep inferior epigastric artery perforator flap breast reconstruction and DIEP flap breast reconstruction coupled with vascularized lymph node transfer: Improving the quality of life of patients with breast cancer related lymphedema without affecting donor site outcomes. Microsurgery 2023;43:213-21. [Crossref] [PubMed]
- Yoshimatsu H, Karakawa R, Fuse Y, et al. Simultaneous Lymphatic Superficial Circumflex Iliac Artery Perforator Flap Transfer from the Zone 4 Region in Autologous Breast Reconstruction Using the Deep Inferior Epigastric Artery Perforator Flap: A Proof-of-Concept Study. J Clin Med 2022;11:534. [Crossref] [PubMed]
- Schaverien MV, Chang EI. Combined deep inferior epigastric artery perforator flap with vascularized groin lymph node transplant for treatment of breast cancer-related lymphedema. Gland Surg 2021;10:460-8. [Crossref] [PubMed]
- Coroneos CJ, Asaad M, Wong FC, et al. Outcomes and technical modifications of vascularized lymph node transplantation from the lateral thoracic region for treatment of lymphedema. J Surg Oncol 2022;125:603-14. [Crossref] [PubMed]
- Inbal A, Teven CM, Chang DW. Latissimus dorsi flap with vascularized lymph node transfer for lymphedema treatment: Technique, outcomes, indications and review of literature. J Surg Oncol 2017;115:72-7. [Crossref] [PubMed]
- Schaverien MV, Badash I, Patel KM, et al. Vascularized Lymph Node Transfer for Lymphedema. Semin Plast Surg 2018;32:28-35. [Crossref] [PubMed]
- Chang EI, Chu CK, Hanson SE, et al. Comprehensive Overview of Available Donor Sites for Vascularized Lymph Node Transfer. Plast Reconstr Surg Glob Open 2020;8:e2675. [Crossref] [PubMed]
- Maldonado AA, Chen R, Chang DW. The use of supraclavicular free flap with vascularized lymph node transfer for treatment of lymphedema: A prospective study of 100 consecutive cases. J Surg Oncol 2017;115:68-71. [Crossref] [PubMed]
- Teven CM, Hunter CL, Chang DW. Management of High-Output Chyle Leak after Harvesting of Vascularized Supraclavicular Lymph Nodes. Plast Reconstr Surg 2019;143:1251-6. [Crossref] [PubMed]
- Roka-Palkovits J, Lin MC, Tzou CJ, et al. Retrograde Manual Lymphatic Drainage following Vascularized Lymph Node Transfer to Distal Recipient Sites for Extremity Lymphedema: A Retrospective Study and Literature Review. Plast Reconstr Surg 2021;148:425e-436e. [Crossref] [PubMed]
- Ward J, King I, Monroy-Iglesias M, et al. A meta-analysis of the efficacy of vascularised lymph node transfer in reducing limb volume and cellulitis episodes in patients with cancer treatment-related lymphoedema. Eur J Cancer 2021;151:233-44. [Crossref] [PubMed]
- Brazio PS, Nguyen DH. Combined Liposuction and Physiologic Treatment Achieves Durable Limb Volume Normalization in Class II-III Lymphedema: A Treatment Algorithm to Optimize Outcomes. Ann Plast Surg 2021;86:S384-9. [Crossref] [PubMed]
- Ciudad P, Manrique OJ, Bustos SS, et al. Single-stage VASER-assisted liposuction and lymphatico-venous anastomoses for the treatment of extremity lymphedema: a case series and systematic review of the literature. Gland Surg 2020;9:545-57. [Crossref] [PubMed]
- Gabriele G, Nigri A, Chisci G, et al. Combination of Supramicrosurgical Lymphatico-Venular Anastomosis (sLVA) and Lymph-Sparing Liposuction in Treating Cancer-Related Lymphedema: Rationale for a Regional One-Stage Approach. J Clin Med 2024;13:2872. [Crossref] [PubMed]
- Masia J, Pons G, Nardulli ML. Combined Surgical Treatment in Breast Cancer-Related Lymphedema. J Reconstr Microsurg 2016;32:16-27. [Crossref] [PubMed]
- Chang DW. Combined Approach to Surgical Treatment of Lymphedema. Lymphat Res Biol 2021;19:23-4. [Crossref] [PubMed]
- Chung JH, Hwang YJ, Park SH, et al. Preliminary outcomes of combined surgical approach for lower extremity lymphedema: supraclavicular lymph node transfer and lymphaticovenular anastomosis. J Plast Surg Hand Surg 2022;56:261-9. [Crossref] [PubMed]
- Mihara M, Zhou HP, Hara H, et al. Case report: a new hybrid surgical approach for treating mosaic pattern secondary lymphedema in the lower extremities. Ann Vasc Surg 2014;28:1798.e1-6. [Crossref] [PubMed]
- Gasteratos K, Morsi-Yeroyannis A, Vlachopoulos NC, et al. Microsurgical techniques in the treatment of breast cancer-related lymphedema: a systematic review of efficacy and patient outcomes. Breast Cancer 2021;28:1002-15. [Crossref] [PubMed]
- Martini F, Meroni M, Scaglioni MF. Pedicled SCIP-based vascularized lymphnode and lymphatic vessels transfer (VLNT and VLVT) for deep lymphatic system reconstruction and dead space obliteration after medial thigh sarcoma resection: A case report. Microsurgery 2024;44:e31205. [Crossref] [PubMed]
- Mihara M, Tange S, Hara H, et al. Modified lymph vessel flap transplantation for the treatment of refractory lymphedema: A case report. Microsurgery 2016;36:695-9. [Crossref] [PubMed]
- Chen WF, McNurlen M, Ding J, et al. Vascularized lymph vessel transfer for extremity lymphedema - Is transfer of lymph node still necessary? Int Microsurg J 2019;3:1. [Crossref]
- Lin WC, Safa B, Buntic RF. Approach to Lymphedema Management. Semin Plast Surg 2022;36:260-73. [Crossref] [PubMed]
- Yamamoto T, Yamamoto N, Kageyama T, et al. Lymph-interpositional-flap transfer (LIFT) based on lymph-axiality concept: Simultaneous soft tissue and lymphatic reconstruction without lymph node transfer or lymphatic anastomosis. J Plast Reconstr Aesthet Surg 2021;74:2604-12. [Crossref] [PubMed]
- Brorson H, Svensson H. Liposuction combined with controlled compression therapy reduces arm lymphedema more effectively than controlled compression therapy alone. Plast Reconstr Surg 1998;102:1058-67; discussion 1068. [Crossref] [PubMed]
- Bustos VP, Friedman R, Pardo JA, et al. Tracking Symptoms of Patients With Lymphedema Before and After Power-Assisted Liposuction Surgery. Ann Plast Surg 2023;90:616-20. [Crossref] [PubMed]
- Hoffner M, Bagheri S, Hansson E, et al. SF-36 Shows Increased Quality of Life Following Complete Reduction of Postmastectomy Lymphedema with Liposuction. Lymphat Res Biol 2017;15:87-98. [Crossref] [PubMed]
- Granzow JW, Soderberg JM, Dauphine C. A novel two-stage surgical approach to treat chronic lymphedema. Breast J 2014;20:420-2. [Crossref] [PubMed]
- Miller TA. Charles procedure for lymphedema: a warning. Am J Surg 1980;139:290-2. [Crossref] [PubMed]
- Kim DI, Huh SH, Hwang JH, et al. Excisional surgery for chronic advanced lymphedema. Surg Today 2004;34:134-7. [Crossref] [PubMed]
- Losco L, Bolletta A, de Sire A, et al. The Combination of Lymph Node Transfer and Excisional Procedures in Bilateral Lower Extremity Lymphedema: Clinical Outcomes and Quality of Life Assessment with Long-Term Follow-Up. J Clin Med 2022;11:570. [Crossref] [PubMed]
- Hassan K, Chang DW. The Charles Procedure as Part of the Modern Armamentarium Against Lymphedema. Ann Plast Surg 2020;85:e37-43. [Crossref] [PubMed]
- Homans J. The Treatment of Elephantiasis of the Legs — A Preliminary Report. N Engl J Med 1936;215:1099-104. [Crossref]
- Mousavi SR. Long Term Results of Innovative Procedure in Surgical Management of Chronic Lymphedema. Open Orthop J 2016;10:543-9. [Crossref] [PubMed]
- Ciudad P, Sabbagh MD, Agko M, et al. Surgical Management of Lower Extremity Lymphedema: A Comprehensive Review. Indian J Plast Surg 2019;52:81-92. [Crossref] [PubMed]
- Ciudad P, Agko M, Huang TCT, et al. Comprehensive multimodal surgical treatment of end-stage lower extremity lymphedema with toe management: The combined Charles,' Homan's, and vascularized lymph node transfer (CHAHOVA) procedures. J Surg Oncol 2019;119:430-8. [Crossref] [PubMed]
- Sapountzis S, Ciudad P, Lim SY, et al. Modified Charles procedure and lymph node flap transfer for advanced lower extremity lymphedema. Microsurgery 2014;34:439-47. [Crossref] [PubMed]
- Dean LT, Moss SL, Ransome Y, et al. "It still affects our economic situation": long-term economic burden of breast cancer and lymphedema. Support Care Cancer 2019;27:1697-708. [Crossref] [PubMed]
- Weiss R. Cost of a lymphedema treatment mandate - 16 years of experience in the Commonwealth of Virginia. Health Econ Rev 2022;12:40. [Crossref] [PubMed]
- De Vrieze T, Nevelsteen I, Thomis S, et al. What are the economic burden and costs associated with the treatment of breast cancer-related lymphoedema? A systematic review. Support Care Cancer 2020;28:439-49. [Crossref] [PubMed]
- H.R.3630 - 117th Congress (2021-2022): Lymphedema Treatment Act | Congress.gov | Library of Congress.
- Tom AR, Boudiab E, Issa C, et al. Single Center Retrospective Analysis of Cost and Payments for Lymphatic Surgery. Plast Reconstr Surg Glob Open 2021;9:e3630. [Crossref] [PubMed]
- La-Anyane O, Alba BE, Harmon KA, et al. United States insurance coverage of immediate lymphatic reconstruction. J Surg Oncol 2024;129:584-91. [Crossref] [PubMed]
- Finkelstein ER, Ha M, Hanwright P, et al. A critical analysis of American insurance coverage for imaging and surgical treatment of lymphedema. J Vasc Surg Venous Lymphat Disord 2022;10:1367-75. [Crossref] [PubMed]
- Rochlin DH, Sheckter CC, Brazio PS, et al. Commercial Insurance Rates and Coding for Lymphedema Procedures: The Current State of Confusion and Need for Consensus. Plast Reconstr Surg 2024;153:245-55. [PubMed]
- Johnson AR, Otenti D, Bates KD, et al. Creating a Policy for Coverage of Lymphatic Surgery: Addressing a Critical Unmet Need. Plast Reconstr Surg 2023;152:222-34. [Crossref] [PubMed]
Cite this article as: Zurbuchen EA, Yu N, Salibian AA. Modern approaches to lymphatic surgery: a narrative review. Transl Breast Cancer Res 2025;6:6.




