
Underlying factors that affect ultrasound conspicuity of breast biopsy markers: an exploratory study
Highlight box
Key findings
• Ultrasound viewing angle can greatly affect clip conspicuity.
What is known and what is new?
• Clips can become inconspicuous on ultrasound scans during neoadjuvant therapy.
• Using ultrasound physics, we investigate potential causes for loss of conspicuity, which may be common to all clips regardless of ultrasound system and settings.
What is the implication, and what should change now?
• Small changes to viewing angle between imaging sessions due to probe positioning and morphological changes due to treatment may affect clip conspicuity. If the probe cannot be physically moved to a more optimal position, other imaging settings may not be able to make clips more conspicuous and other imaging modalities may need to be considered.
Introduction
Background
As part of the standard of care for using neoadjuvant systemic therapy (NST) to treat breast cancers, breast biopsy clips or markers are implanted into the target areas prior to treatment with NST (1,2). These markers are designed to improve identification of the treated areas after NST, but it has been reported that in up to half of preoperative ultrasound scans these clips are no longer visualized (3,4). Because ultrasound is often used to guide insertion of localizers placed near the site of the clips and used during surgery, visual confirmation of the locations of the clips on ultrasound scans is critical.
Advancements in clip technology and ultrasound image quality have improved the ability to observe clips in vivo since their inception. Breast biopsy clips have become increasingly larger as materials such as nitinol have been integrated into designs to facilitate the clips returning to more elaborate shapes as they exit the needle into the tumor or lymph node site (5). Furthermore, most non-nitinol clips are packed into the deploying needles with biocompatible embedding materials that are intended to reduce migration and increase the acoustic echogenicity of these clips, particularly during insertion.
Rationale and knowledge gap
Many studies on clips have been carried out to determine their overall efficacy for mammographic, magnetic resonance imaging, and sonographic marking and dependence, if any, on factors such as clip type (6,7), timing of placement (8), staging of lesion (1), or additional effects such as color Doppler twinkling for some types of clips (9-11). Sonographic variability in visualization can be attached to particularly over or under-performing clip types, and many clips are compatible with other modalities that can confirm placement (metallic clips, in particular, are radiopaque). However, breast imaging practices typically do not purchase all clip types and generally resort to practice consensus on the type of clip appropriate for their use. There are some general guidelines for adjusting imaging settings to improve the sonographic conspicuity for any given clip (12), but equipment capabilities may vary from vendor to vendor. So far, an understanding of mechanisms behind conspicuity changes is not well understood.
Objective
In this work, we approach this problem from an ultrasound physics perspective to better appreciate what may be happening in cases where the clips lose sonographic conspicuity and become difficult to distinguish against tissue background. We first review the relevant basic theory on the propagation of ultrasound in any given material, which in turn directly affects the contrast of objects in ultrasound. We also explore how transducer orientation or imaging angle may affect the conspicuity of clips by scanning several commercially available clips at different orientations relative to an imaging array probe. This simulates what occurs during clinical scanning as the underlying malignancy or lymph node undergoes morphological changes in response to NST, and where the position of the probe relative to the imaged clips may vary between imaging sessions.
Clip conspicuity is complicated by the numerous ultrasound scanner settings that can be adjusted, such as focus, center frequency, beam steering, and dynamic range. Determining the optimal settings and combinations to acquire the highest-quality scan of clip features is nontrivial. However, understanding the underlying physical principles affecting conspicuity can help identify which settings, if any, might be most effective. If no settings can improve visualization, knowing this would save time by avoiding unnecessary adjustments. Furthermore, resources could be more efficiently redirected toward finding clips using other imaging modalities.
We further hypothesize that some biological processes may be involved in the observed degradation of conspicuity over time for some types of radiological clips (7), e.g., off-gassing or release of small, attached air bubbles from a dry clip injected into a tissue environment. To test this hypothesis, we interrogated an ex vivo sample with two embedded clips to assess conspicuity as a function of time. While a clip in an ex vivo sample may or may not display any conspicuity changes, it can be used to help rule out some processes affecting conspicuity as either inherent to ultrasound physics or a result of biological changes that occur in living tissue.
Generating contrast in ultrasound
Contrast in ultrasound is generated from scattering and reflection within the heterogeneous material being imaged. In the simple case of a compressible sphere of different material than its homogeneous surroundings, radiation pressure (scattering) can be estimated as:
where instantaneous pressure at distance r from the sphere and viewing angle θ is dependent on initial pressure , wavenumber k, sphere radius a, the compressibility of the sphere () and its containing medium (), and the densities of the sphere and medium, and , respectively. Of particular interest are the compressibility and density terms. Although clips are not typically spheres like the equation models, contrast is still dependent on these material properties, which remain the same regardless of shape and distance.
In the case of an air bubble contained within water, is approximately 15,500. For a titanium sphere [ρTi≈4,500 kg/m2, cTi=6,070 m/s (13)], this value is 0.0632 when cosθ =1. Air bubbles thus scatter substantially more than titanium.
Reflection plays a larger role in the brightness of flat clips in ultrasound, where the ratio R of reflected pressure to incident pressure is given by
assuming that the incident pressure wave meets the boundary at normal incidence. Here, a water-titanium boundary produces |R| =0.896, and a water-air boundary produces |R| ≈1. Thus, air is also a greater reflector of acoustic pressure. If an incident wave is not at normal incidence upon the boundary, i.e., oblique incidence, the reflected pressure wave may not be received in its entirety by the imaging probe. This further reduces boundary brightness, as shown in Figure 1.
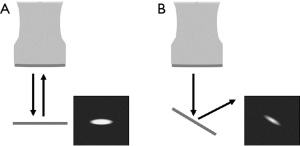
As a result of its large impedance mismatch with water and high compressibility, it is expected that air pockets will produce the highest return pressure and thus the brightest signal in a B-mode image, particularly compared to titanium and other clips with similar metallic properties. However, it may be possible for small air molecules to attach to a metallic clip upon insertion or be present in a needle track, which would theoretically increase brightness (and likely conspicuity) of a clip at the time of insertion.
It is worth noting that brightness is not the only factor that affects conspicuity, and it is not necessarily sufficient on its own to provide conspicuity. Conspicuity can depend on additional factors, e.g., the contrast of the clip with the surrounding areas, the shape of the bright spot associated with the clip, and changes of the clip signal with probe motion. However, brightness is a useful starting metric and the most readily influenced by ultrasound system settings, hence the role of brightness/contrast as a proxy metric for assessing conspicuity in this work.
Methods
To investigate the effect of viewing angle on clips, a series of 12%-gelatin tissue-mimicking phantoms were cast in an icosahedral mold (approximately 23 mm diameter). Gelatin was chosen for ease of release from the mold and ease of placement of clips removed from their embedding material. Investigated clips included the M, omega, and eye (Hologic®, Marlborough, MA, USA), and the barrel and open coil (Mammotome, Cincinnati, OH, USA), all shown in Figure 2. An additional phantom with an approximately spherical 3-mm-diameter air bubble at its center was imaged, and based on measurements of this air bubble, a seventh and final phantom with a 3-mm-diameter titanium bead (Micro Surface Engineering, Inc., Los Angeles, CA, USA) was also imaged. Each phantom was imaged with a SonixOne system using an L14-5/30 probe through a degassed water standoff of less than 5 mm. A total of 100 frames of beamformed data were gathered from the fixed imaging probe for each of the 20 sides of the icosahedral phantoms (see Figure 3). The probe was swept across each face of the phantom to find the plane where the clip was visually brightest and then fixed there to record data.

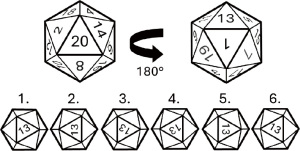
The phantoms could be rotated in the lateral-elevational plane to produce a slightly different image for each side, and the icosahedral mold shape naturally formed six discrete rotations per side (with rotations in approximately 60° increments), as shown in Figure 3. Three trials were acquired for each rotation, and each rotation was treated as an independent imaging session. In total, 18 independent scans were acquired for each of the 20 sides for each of the phantoms studied. Because of the natural symmetry of the phantoms at opposing rotations, data for Rotations 1 and 4, 2 and 5, and 3 and 6 were combined in post-processing. The resulting data set consisted of three rotational views with six independent trials each for the 20 sides of every phantom.
The clips were placed against a very low-noise background, so contrast-to-noise ratio (CNR) did not capture the qualitatively-observed patterns in clip brightness. We thus devised a metric we call “mean maximum brightness” (MMB) to assess the brightness of the clips intuitively and quantitatively. To calculate the MMB for a given scan, a region of interest (ROI) measuring 1 mm square (approximately 8 by 54 datapoints) was manually placed over the visually brightest portion of the clip in an image frame. The 100 frames of data for each scan were averaged together to reduce electronic noise and account for aberrations in a given frame. The 10 brightest datapoints in this frame-averaged ROI were averaged together to produce a single MMB value for a given side of a phantom. Up to 432 datapoints comprised the 1-mm-by-1-mm ROI. The number of averaged datapoints in the MMB was adjustable up to this value, i.e., averaging the brightness of the entire ROI, but averaging 10 points was found to best represent the brightest regions of both large and small bright spots.
A sample of pork belly with striations of fat and muscle was purchased at a local supermarket and cut into a strip measuring approximately 14 cm long, 2 cm thick, and 3.5 cm deep. These dimensions were aligned with the probe in the lateral, elevational, and axial dimensions, respectively. The open coil in its polyethylene glycol hydrogel embedding material and the barbell in its beta glucan embedding material were inserted into this sample using ultrasound guidance from an L14-5/30 probe. The insertion sites were approximately centered in the sample elevationally, with the final positions being approximately 2 cm deep for the open coil and 1 cm deep for the barbell. Approximate locations within the meat sample are shown in Figure 4. Deployment of the clips was recorded and their position imaged both from the top of the sample and from the side. This sample was imaged during injection (day 0) and every 3 to 4 days thereafter for 56 days and a total of 17 imaging sessions.

Background tissue scattering had a significant effect ex vivo, unlike in the phantoms. CNR was thus used to assess the brightness of the clips in the sample to determine if there were any changes over the course of the imaging period. CNR was calculated as:
where µin and µout indicate the mean value of the signal strength inside the clip ROI and the background ROI, respectively, and and are the variances of the signal strength for the ROIs drawn inside and outside the clip (14).
Three ROIs measuring 1 mm by 1 mm were selected and averaged together to obtain a CNR value for a given day, view, and clip. A second set of three ROIs were drawn to calculate CNR of confounding scatterers. These scatterers were selected on an image-by-image basis, depending on what was visible in the imaging field that could be reasonably confused for the clip signal.
Results
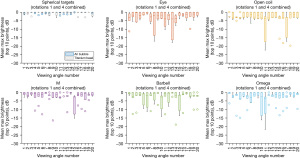
The air bubble and titanium bead were found to have the highest MMB (over n=6 trials) compared to all commercial clips tested, as shown in Figure 5. When viewed from sides 7 and 14 (opposing sides on the phantom), the flat M clip had a similar MMB to the air bubble, with its MMB surpassing the air bubble’s MMB by 0.1 dB for side 7. The eye clip viewed from side 6 was similarly able to approach within 0.1 dB of the air bubble for the same side. Each clip had at least one angle with a MMB of greater than −1 dB, and all clips had at least one angle with a MMB of less than −5 dB. The range of MMB can provide an initial assessment of the variation between angles, with a larger range suggesting a larger difference between sides. The two spherical targets had the smallest range for their MMB across all viewing angles, with the air bubble having a range of 0.349 dB and the bead having a range of 1.07 dB. Of the clips, the open coil had the largest MMB range at 7.37 dB, and the barbell had the smallest range at 4.84 dB.
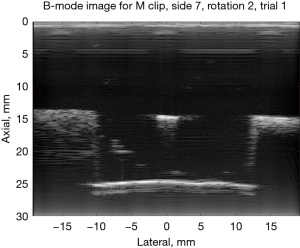
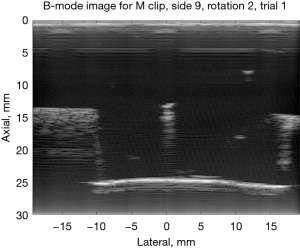
B-mode images are shown for the M clip as viewed from sides 9 and 7 to illustrate what these MMB differences correspond to in B-mode scans. In Figure 6, side 7 for the M clip is shown; it appears as a bright line in the center of the image and is parallel to the imaging probe both laterally and elevationally. The MMB for scans of side 7 had a mean of −0.264 dB. In Figure 7, the “M” shape is tilted such that it is approximately perpendicular to the imaging plane, and the three prongs of the bottom of the “M” shape are in-plane. Here, the MMB was estimated at −4.14 dB.
In Figure 8, 6 datapoints per angle clarify the relationship between viewing angle and MMB. Rotations 1 and 4 are displayed, but the data from rotations 2 and 5 and rotations 3 and 6 were similar. These graphs are displayed at a fixed scale from −30 to 0 dB, with detail shown in Figure 9 for the spherical targets at a scale of −3.5 to 0 dB. MMBs for individual viewing angles for the clips generally had much wider inter-quartile ranges than those for the air bubble and bead. The eye clip had the loosest clustering in its measurements, particularly for angles 10 and 11. The M clip had some of the tightest measurement clustering: from side 12, the clip appeared very bright over a relatively large portion of its shape. The open coil had notably consistent inter-quartile ranges and few outliers for 14 of its angles, though these tended to be somewhat large at around 4 dB. Comparatively, the air bubble and titanium bead had very little variation in MMB within the data gathered. In Figure 9, the titanium bead appeared to, for angles 1, 2, 8, 10, and 20, have a substantially larger inter-quartile range than the air bubble measurements. For the data acquired ex vivo, CNR of the clips was not found to vary statistically significantly with time for two of the three views. The three views are compared to each other in Figure 10: two for the open coil and one for the barbell. There was a data recording issue for the barbell on day 16, resulting in a break in its plot. Using a linear regression model and analysis of variance (ANOVA) testing to compare time and CNR, the P values for the barbell and open coil side view models were 0.438 and 0.360, respectively. For the open coil top view, P=0.01 for a model with R2=0.361 and slope 0.0168.
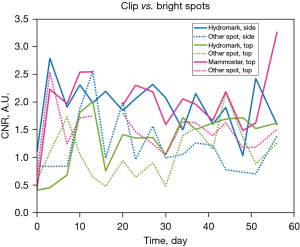
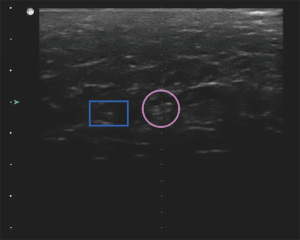
The open coil side view and barbell had relatively similar CNRs, with the top view of the open coil generally having a lower CNR than either of these. Other bright spots in the field of view, indicated with dashed lines in Figure 10, had generally similar CNRs to the respective clip in the image. The chosen confounding scatterers surpassed the CNR of the clip for four of the scans of the open coil top view, while the other two clips had their CNR surpassed by confounding scatterers in two of the scans.
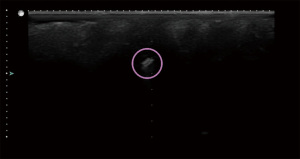
Despite having similar CNRs to confounding scatterers, both the open coil side view and barbell remained consistently conspicuous throughout the imaging period. For the open coil, the background tissue was relatively uniform in the side view and the clip shape was easily identified as shown in Figure 11. The beta glucan encasing the barbell (Figure 12) provided large hypoechoic regions to either side of the clip, making its location more conspicuous. The open coil top view had only a small hypoechoic region likely related to the hydrogel component that was visible (Figure 13) and was not a conspicuous shape, lowering its CNR.

Discussion
Key findings
The results from the viewing angle trials reflect what was anticipated based on the fundamental scattering and reflection equations presented in the “Generating contrast in ultrasound” section. The two spherical reflectors, the air bubble and bead, maintained the highest and most consistent MMB across all scanning angle orientations. Clips that had flatter shapes, such as the M and omega, had MMBs that varied the most between angles, which was also in line with expectations about reflected waves being captured by the probe. From a qualitative perspective, these differences were quite pronounced, even in a plain gelatin phantom with minimal scattering. Combined with other factors such as tissue heterogeneity, access to a limited number of viewing angles with an ultrasound probe, and minute shifts that occur as NST affects tissue morphology, it is plausible that in some cases a sufficient viewing angle may be lost.
This is further corroborated by the ex vivo portion of this study, where the open coil was found to be deployed in a suboptimal orientation relative to the surface of the tissue sample. Because the tissue sample was ex vivo, the sample could be imaged at a plane that was perpendicular to the actual tissue surface, which allowed the open coil to be observed. However, imaging at 90° from the plane of the tissue surface is a strategy that may not always be feasible in a clinical setting. The barbell did not appear to rotate upon deployment in this way, and no further viewing angles were required to successfully locate this clip.
Operator familiarity with the open coil’s location in the sample increased as time progressed, which counteracted the minimal expansion of the hydrogel. Though hydrogel expansion is intended to aid in localization by creating an anechoic region around the clip, it is possible that insufficient temperature or moisture may cause it to not expand outside of in vivo environments like the one we tested. Operator familiarity, rather than hydrogel conspicuity, therefore enabled more successful probe positioning for top-down imaging. This may be the underlying reason for the slight but statistically significant increase in clip CNR with respect to time for the top-view open coil scans. Such a degree of familiarity may not be achievable in a clinical setting, although it is expected that the hydrogel embedding material may expand to a more obvious size.
Given the significant impact of orientation on clip conspicuity, it is easy to assume that a spherical marker would be ideal. However, we caution that spherical titanium beads have similar issues with correct probe positioning as we have found in previous work (15). If the insertion needle size is fixed, spherical beads must be inherently smaller than other clips, which can take more space along the length of the barrel for a larger, potentially brighter target. Spherical metal or other biocompatible beads are also ultimately subject to the physical properties of their composition, which will likely not be able to match the compressibility or reflectivity of air in tissue. The barbell, for example, is a carbon-coated ceramic clip and did not perform differently from the titanium clips. Its density is more akin to a titanium clip than to air, affecting compressibility and reflectivity regardless of its sound speed.
In these experiments, we found minimal performance differences between the air bubble and the titanium bead. Theory suggests there should be some differences due to increased scattering from the air bubble. Both the air bubble and spherical bead exhibited signal saturation with the chosen imaging settings. Because the other clips did not saturate and the air bubble was considered a theoretical upper limit on MMB, allowing signal saturation for the air bubble and bead—the two most uniformly-shaped targets—was considered acceptable.
The titanium bead, despite being a highly spherical object, had some viewing angles with unexpectedly large inter-quartile ranges. As the phantoms were refrigerated for storage and imaged at room temperature in water, expansion of the phantom created a small air pocket around parts of the bead in later imaging sessions. This may have contributed to nonuniformity in the bead response.
In clinical practice, maximum imaging depth in ultrasound is primarily dictated by body habitus, the tissue structure overlying the targeted area, and probe transmit frequency. Depths of 6 cm or more are achievable in the breast, when needed. In the context of marked breast lesions and lymph nodes, ultrasound depth from skin surface to targeted marker ranges from about 0.4 to 2.5 cm and averages around 1 cm (16). The targeted depths in our experiments, both in phantom and ex vivo, closely align with these observed values.
Strengths and limitations
This study lacks in vivo data and tests only a relatively small number of clip designs, which we consider to be weaknesses that limit our ability to study the phenomenon of lost conspicuity with the highest granularity.
However, a novel approach to phantom creation allowed clips to be imaged from 20 different angles to confirm the effects of the simple act of probe positioning on the conspicuity of multiple commercial markers. Further investigation could entail adjustments to settings while keeping the probe and clip positioning fixed, the study of additional clips, or collaboration with institutions to acquire and process human data using the same methods herein.
Conclusions
Breast biopsy clip conspicuity remains an issue in breast cancer treatments involving NST, as the clips may become indistinct after favorable response to NST. This lack of visual clarity can create challenges during imaging, and understanding the underlying causes to identify solutions could reduce imaging time and stress for both patients and sonographers. To circumvent issues with ultrasound conspicuity, clinics may wish to purchase other localizers with proprietary probes and non-ultrasound localization methods such as the Savi Scout (radar) or a LOCalizer (radiofrequency identification) tagging system (16,17). Such localizers generally occupy a different use case and are not necessarily designed for compatibility in ultrasound scans, but these markers can provide better localization than ultrasound. New clip designs that address current shortcomings could also address this clinical need, such as an ultrasound-compatible electronic clip design that we previously proposed (18). Even as practice changes evolve from results of ongoing clinical trials investigating the best way to manage node-positive breast cancer, breast biopsy markers will almost certainly continue to play a critical role.
In this work, we investigated how basic ultrasound physics plays a role in the loss of clip conspicuity, particularly with concern to viewing angle and material properties. We found that viewing angle can have a dramatic effect on clip conspicuity, but caution that this issue is present in nearly all clips. Spherical bead markers may not have angle orientation issues, but they are subject to the same issues of reflection that govern the conspicuity of other clip types from various viewing angles.
Injecting clips into meat to examine their behavior with respect to time showed consistent conspicuity in our trials, suggesting that any additional phenomena that may affect conspicuity over the duration of NST are not inherent to the clips themselves, and may be a result of biological processes. No evidence of off-gassing was observed with the inserted clips, and we attribute CNR changes with time to be the result of imaging technique. We do caution, however, that the clips can change appearance between deployment and the following time points as their embedding material reaches its intended state. The presence of other bright scatterers in the field can obscure the ability to observe actual clips in real tissues, which can mimic the appearance of clips.
Acknowledgments
None.
Footnote
Data Sharing Statement: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-48/dss
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-48/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-48/coif). C.U.L. serves as an unpaid editorial board member of Translational Breast Cancer Research from October 2023 to September 2025. J.C. reports that the study was supported by the Cancer Scholars for Translational and Applied Research (C⋆STAR) Program sponsored by the Cancer Center at Illinois and the Carle Cancer Center under Award Number CST EP082021. M.L.O. reports this study was funded in part by grants from the National Institutes of Health (NIH) (Nos. R21EB030743, R01CA251939, and R01CA273700). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nguyen TT, Hieken TJ, Glazebrook KN, et al. Localizing the Clipped Node in Patients with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy: Early Learning Experience and Challenges. Ann Surg Oncol 2017;24:3011-6. [Crossref] [PubMed]
- Alshafeiy TI, Matich A, Rochman CM, et al. Advantages and Challenges of Using Breast Biopsy Markers. J Breast Imaging 2022;4:78-95. [Crossref] [PubMed]
- Hyde B, Geske J, Lee C. Challenges to I-125 Seed Localization of Metastatic Axillary Lymph Nodes Following Neoadjuvant Chemotherapy. J Breast Imaging 2019;1:223-9. [Crossref] [PubMed]
- Taj R, Chung SH, Goldhaber NH, et al. Localizing Positive Axillary Lymph Nodes in Breast Cancer Patients Post Neoadjuvant Therapy. J Surg Res 2023;283:288-95. [Crossref] [PubMed]
- Shah AD, Mehta AK, Talati N, et al. Breast tissue markers: Why? What's out there? How do I choose? Clin Imaging 2018;52:123-36. [Crossref] [PubMed]
- Koo JH, Kim EK, Moon HJ, et al. Comparison of breast tissue markers for tumor localization in breast cancer patients undergoing neoadjuvant chemotherapy. Ultrasonography 2019;38:336-44. [Crossref] [PubMed]
- Pinkney DM, Shah BA. Prospective Comparative Study to Evaluate the Sonographic Visibility of Five Commercially Available Breast Biopsy Markers. Journal of Diagnostic Medical Sonography 2013;29:151-8. [Crossref]
- Balija TM, Braz D, Hyman S, et al. Early reflector localization improves the accuracy of localization and excision of a previously positive axillary lymph node following neoadjuvant chemotherapy in patients with breast cancer. Breast Cancer Res Treat 2021;189:121-30. [Crossref] [PubMed]
- Tan MP, Bi Z, Ong EMW. The 'twinkle' artifact - A novel method of clip identification to facilitate targeted axillary surgery following neoadjuvant chemotherapy in breast cancer patients. Clin Imaging 2020;68:36-44. [Crossref] [PubMed]
- Lee CU, Hesley GK, Uthamaraj S, et al. Using Ultrasound Color Doppler Twinkling to Identify Biopsy Markers in the Breast and Axilla. Ultrasound Med Biol 2021;47:3122-34. [Crossref] [PubMed]
- Lee CU, Larson NB, Urban MW, et al. Factors Associated With Ultrasound Color Doppler Twinkling by Breast Biopsy Markers: In Vitro and Ex Vivo Evaluation of 35 Commercially Available Markers. AJR Am J Roentgenol 2023;220:358-70. [Crossref] [PubMed]
- Lee C, Zhou C, Hyde B, et al. Techniques for Improving Ultrasound Visualization of Biopsy Markers in Axillary Lymph Nodes. J Clin Imaging Sci 2020;10:21. [Crossref] [PubMed]
- Rumble JR. editor. CRC Handbook of Chemistry and Physics [Internet]. 102nd ed. CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press/Taylor & Francis; 2021. Available online: https://hbcp.chemnetbase.com/faces/documents/14\_19/14\_19\_0001.xhtml
- Patterson MS, Foster FS. The improvement and quantitative assessment of B-mode images produced by an annular array/cone hybrid. Ultrason Imaging 1983;5:195-213. [Crossref] [PubMed]
- Cario J, Coila A, Zhao Y, et al. Identifying and overcoming limitations with in situ calibration beads for quantitative ultrasound. J Acoust Soc Am 2022;151:2701. [Crossref] [PubMed]
- Falcon S, Weinfurtner RJ, Mooney B, et al. SAVI SCOUT® localization of breast lesions as a practical alternative to wires: Outcomes and suggestions for trouble-shooting. Clin Imaging 2018;52:280-6. [Crossref] [PubMed]
- Lowes S, Bell A, Milligan R, et al. Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: experience of the first 150 cases in a UK breast unit. Clin Radiol 2020;75:942-9. [Crossref] [PubMed]
- Cario J, Kou Z, Miller RJ, et al. A Radiological Clip Design Using Ultrasound Identification to Improve Localization. IEEE Trans Biomed Eng 2024;71:2699-707. [Crossref] [PubMed]
Cite this article as: Cario J, Lee CU, Oelze ML. Underlying factors that affect ultrasound conspicuity of breast biopsy markers: an exploratory study. Transl Breast Cancer Res 2025;6:2.




