
Advances in antibody-drug conjugates in the treatment of advanced triple-negative breast cancer: a narrative review
Introduction
Background
Breast cancer is the most common malignant tumor to threaten women’s health. Approximately, 3–10% of new cases of breast cancer have distant metastasis at the time of diagnosis every year, whereas 30% of early-stage breast cancer eventually develop advanced breast cancer (1). Advanced triple-negative breast cancer (aTNBC) lacks the expression of the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) (2), resulting in low sensitivity to endocrine therapy and HER2-targeted therapy. The treatment options available are limited. The main treatment method is traditional chemotherapy, but the efficacy is limited and the adverse reaction is severe (3), leading to death in nearly 70% of patients with breast cancer (4).
In recent years, with the exploration and application of biological characteristics and related biomarkers of TNBC, significant progress has been made in drug research and development for the treatment of aTNBC. Moreover, research achievements such as targeted therapy and immunotherapy have expanded the range of available treatment options for patients with aTNBC. In particular, the success of antibody-drug conjugates (ADCs) has established a new clinical treatment model for aTNBC, redefining not only the clinical treatment practice of aTNBC but also the treatment prospects of metastatic breast cancer (5,6).
Objective
The goal of this article is to provide a comprehensive review and analysis of the advances of ADCs for aTNBC, and also discusses clinical significance and future research directions of ADCs in the future. We present this article in accordance with the Narrative Review reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-38/rc).
Methods
As of December 2023, a comprehensive literature search and analysis were conducted across PubMed, Wanfang Data, ClinicalTrials.gov, and relevant academic conferences, to identify the latest published literature or ongoing trials on ADCs for aTNBC. The selected literature primarily focused on the drug structural profile, pharmacological mechanism, important trials targeting different antigens, and other exploratory investigations (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | November 15, 2023 to December 31, 2023 |
| Database searched | PubMed, Wanfang Data, ClinicalTrials.gov, and relevant academic conferences (such as the San Antonio Conference) |
| Search terms used | Triple-negative breast cancer; metastatic triple-negative breast cancer; antibody-drug conjugate; trophoblast cell-surface antigen 2; human epidermal growth factor receptor 2; targeted therapies |
| Timeframe | Past 10 years |
| Inclusion and exclusion criteria | Inclusion criteria: our selection primarily includes latest research articles, reviews, and clinical trials published in English or Chinese. The focus is mainly on drug structural profile, pharmacological mechanism, important trials targeting different antigens, and other exploratory investigations |
| Exclusion criteria: papers in other language, or not relevant to triple-negative breast cancer | |
| Selection process | Most of the literature was selected by the author K.J., supplemented by the second author S.W., and reviewed by both authors |
| Any additional considerations | While reviewing the relevant literature, some cited references were also taken into account |
Brief introduction of previous studies in TNBC
Clinical and epidemiological characteristics of TNBC
In China, TNBC accounts for approximately 10.4–13.5% of patients with breast cancer. The clinical characteristics of TNBC include an early-onset, large tumor, high axillary lymph node positive rate and a higher tumor histological grade, which directly increase the risk of metastasis and poor prognosis (7). The 5-year relative survival rate was 93% for other breast cancer subtypes, 77% for TNBC, and only 14% for aTNBC (8). A long-term follow-up of patients with unilateral new breast cancer showed that the distant metastasis rate following surgery for TNBC was 25.3% (9), and the median cancer-specific survival time of metastatic TNBC (mTNBC) was only 12 months (10). Among them, the treatment of “refractory aTNBC” is particularly difficult, which may be characterized by early recurrence of cancer and high drug resistance, that is, disease-free survival ≤12 months and disease progression after drug replacement, or poor tolerance of the first-line treatment regimen, a high incidence of treatment-related adverse events (TRAEs), or drug discontinuation (11). Most clinical trials exclude patients with refractory aTNBC, affecting the potential clinical benefit for the relevant patients. Consequently, TNBC is a more threatening disease than hormone receptor-positive (HR+) and/or human epidermal growth factor receptor 2-positive (HER2+) breast cancer subtypes. It is currently a tough challenge in clinical treatment as well as a key research topic.
Advances in TNBC treatment in the pre-ADCs era
Chemotherapy is the most important treatment option for aTNBC, and its recent progress is mainly reflected in the optimization of drug selection, sequencing, dosage, and regimen adjustment (5). However, studies have shown that for patients with aTNBC receiving second or later lines of treatment, the median progression-free survival (PFS) of a single-agent chemotherapy is <3 months and the objective response rate (ORR) is only 11% (12).
The immune checkpoint inhibitor (ICI) can reverse the tumor-mediated suppression of immune cell function and has become one of the important options for tumor-related immunotherapy (13). In TNBC, the most investigated ICI targets are programmed death protein-1 (PD-1) and programmed cell death protein-ligand 1 (PD-L1). The Keynote-119 study reported that pembrolizumab single-agent did not significantly improve the survival rate compared with chemotherapy in patients with previously treated TNBC (14). Nevertheless, in patients with PD-L1-positive TNBC, chemotherapy combined with atezolizumab or pembrolizumab may enhance efficacy, particularly in patients with strong PD-L1 expression may benefit more significantly. However, the majority of patients treated with ICI develop acquired resistance (15), and subsequent treatment remains a challenge.
The BRCA1/2 gene is a principal signaling pathway for the repair of DNA. Mutations in the BRCA1/2 gene occur in approximately 10% of patients with TNBC, which is more aggressive and difficult to treat (16). The synthetic lethality of BRCA1/2 gene mutations with poly (ADP-ribose) polymerase (PARP) inhibitors has led to the development of a treatment for advanced breast cancers associated with germline BRCA gene mutations. The studies of olaparib (17) and talazoparib (18) enrolled a certain number of patients with TNBC having germline BRCA1/2 gene mutations, both of which met the primary study endpoint of prolonging PFS. However, the improvement of overall survival (OS) did not reach statistical significance (19,20), and the clinical use of related drugs in China is still not approved.
In brief, although some progress has been made in chemotherapy, ICI, or targeted PARP therapies for TNBC, the majority of advances to date remain insufficient to meet clinical needs for highly heterogeneous TNBC, especially for patients with refractory aTNBC. This is because of poor efficacy, presence of significant resistance to drug, or the inadequate favorable conditions. With the expansion of new treatment modes, the advent of ADCs has undoubtedly represented a breakthrough in the current treatment dilemma of TNBC.
Main advances in ADCs therapy in TNBC
Mechanism of action and structural characteristics of ADCs
The ADCs consist of three components: monoclonal antibodies targeting tumor antigens, highly potent cytotoxic payloads, and linkers. The ADCs reach the tumor lesion through the bloodstream, where they specifically bind to the target antigen, such as human trophoblast cell-surface antigen 2 (TROP2) and HER2, on the surface of the tumor cell. Subsequently, the ADCs are internalized and fused with lysosomes, where the drug load is released by the enzyme or other chemical action to affect the target effector and finally kill the cancer cells. Combining the advantages of highly targeted antibodies and highly cytotoxic drugs, while retaining the ability of small molecule cytotoxic drugs to kill tumors, results in a high degree of selectivity. This property reduces the off-target toxicity of small molecule cytotoxic drugs and effectively improves the benefit-to-risk ratio of antitumor therapy (21). A meta-analysis of relevant randomized controlled trials reported that ADCs significantly improved PFS, OS, and clinical benefit rate (CBR) in patients with breast cancer compared with chemotherapy drugs or other anti-tumor drugs (22). The ADCs have become one of the key research directions in breast cancer because of their advantages that have greatly changed the traditional treatment strategies for breast cancer.
According to the iterative process of the three components of ADCs, these drugs can be roughly divided into three generations (23): (I) the antibody of the first-generation ADC is a murine or chimeric humanized antibody. The linker is unstable, and the drug load is azithromycin or doxorubicin. The drug-antibody ratio (DAR) is uncontrollable. The disadvantages include high heterogeneity, a narrow therapeutic index, off-target toxicity, and high immunogenicity. (II) The antibody of the second-generation ADCs is a humanized antibody possessing cleavable or non-cleavable linkers with improved stability, and with the drug load including calendulin and maytansine. The DAR of the second-generation ADCs is demonstrably superior to that of the first-generation ADCs, exhibiting enhanced targeting ability and drug-load efficacy while concomitantly reducing immunogenicity. However, drug heterogeneity and off-target toxicity remain. (III) The antibody of the new-generation ADCs is a fully humanized antibody or fab fragment of immunoglobulin G (IgG). The linker is stable in blood circulation while accurately releasing drugs into tumor lesions. Moreover, owing to the “bystander effect”, it still has a strong ability to kill adjacent cancer cells with low or without expression of specific antigens, which is a critical mechanism in the treatment of heterogeneous tumors. The “bystander effect” is influenced by the internalization properties of the ADCs, the cleavability of the linker, and the membrane permeability of the drug load (24). In addition, the drug load is highly cytotoxic, and the homogeneity of the ADCs such as DAR is superior, with a corresponding reduction in off-target toxicity. However, a high cytotoxic drug load may lead to adverse events and may induce drug resistance under certain conditions.
Progress in clinical study of ADCs in aTNBC
Recently, ADCs targeting TROP2 and HER2 have made significant progress against aTNBC, and Table 2 summarizes relevant clinical trials.
Table 2
| Trial name | Sample size | Patients enrolled | Drugs compared | Efficacy profile |
|---|---|---|---|---|
| Targeting TROP2 | ||||
| ASCENT (25,26) | 468 | Refractory or relapsed aTNBC patients with ≥2 lines of chemotherapy | SG vs. TPC | Median PFS: 5.6 vs. 1.7 months; median OS: 12.1 vs. 6.7 months; ORR: 35% vs. 5% |
| EVER-132-001 (27) | 80 | Chinese patients with aTNBC who had received ≥2 lines of standard chemotherapy | SG vs. standard treatment | Tumor size reduction of ≥30% in 50% of patients |
| TROPION-PanTumor01 (28) | 44 | mTNBC patients with median three-line treatment | Dato-DXd vs. none | ORR: 32%; DCR: 80%; median PFS: 4.3 months; median OS: 12.9 months |
| NCT04152499 (29) | 59 | mTNBC patients; 88% had received ≥3 lines of therapy | SKB264 vs. none | ORR: 40%; DCR: 80% |
| Targeting HER2 | ||||
| DESTINY-Breast04 study (29) | 557 | Advanced breast cancer of HER2-low with a median of three lines of prior therapy | T-DXd vs. TPC | PFS: 8.5 vs. 2.9 months; OS: 18.2 vs. 8.3 months; ORR: 50% vs. 16.7% |
| DAISY (30) | 186 | Metastatic breast cancer patients divided into three cohorts: HER2+, HER2-low, and HER2-zero | T-DXd vs. anthracyclines and taxanes | ORR: 37.5% in HER2+ cohort; 29.7% in HER2-low cohort; median PFS: 6.7 months in HER2+ cohort; 4.2 months in HER2-low cohort |
ADCs, antibody-drug conjugates; TROP2, trophoblast cell-surface antigen 2; HER2, human epidermal growth factor receptor 2; aTNBC, advanced triple-negative breast cancer; SG, sacituzumab govitecan; TPC, the physician’s choice of treatment; PFS, progression-free survival; OS, overall survival; ORR, objective response rate; mTNBC, metastatic triple-negative breast cancer; Dato-DXd, datopotamab deruxtecan; DCR, disease control rate; T-Dxd, trastuzumab deruxtecan.
Drugs structure of ADCs targeting TROP2
In TNBC, targeted antigen research on ADCs first made a breakthrough in TROP2, which is a transmembrane glycoprotein expressed by a variety of malignant tumor cells and acts as a tumor-associated calcium signal transducer. The function of this protein is associated with cell migration and anchorage-independent growth relating to prognosis and drug resistance (31-33). TROP2 was found to be highly expressed in only 18.75% of benign breast tumors and 12.5% of normal breast tissues (34), but moderately or robustly expressed in approximately 90% of TNBC (35), correlated with lymph node status, tumor metastasis, tumor node metastasis (TNM) classification, and reduced OS (36). Consequently, TROP2 is regarded as a crucial target for the development of novel ADCs for the treatment of TNBC.
At present, TROP2-targeting ADCs mainly include sacituzumab govitecan (SG), datopotamab deruxtecan (Dato-DXd), and sacituzumab tirumotecan (SKB264). The structural characteristics of these ADCs are presented in Figure 1 and summarized as Table 3.
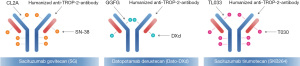
Table 3
| Drugs name | mAb | Linker (feature) | Payload (property) | DAR |
|---|---|---|---|---|
| SG (31) | Humanized IgG1 | CL2A (pH-sensitive) | SN38 (irinotecan-derivative) | 7.6:1 |
| Dato-DXd (37) | Humanized IgG1 | GGFG tetrapeptide (enzyme-sensitive) | DXd (exatecan-derivative) | 4.0:1 |
| SKB264 (38) | Humanized IgG1 | TL033 (pH-sensitive) | T030 (belotecan-derivative) | 7.4:1 |
ADCs, antibody-drug conjugates; TROP2, trophoblast cell-surface antigen 2; TNBC, triple-negative breast cancer; DAR, drug-to-antibody ratio; mAb, monoclonal antibody; SG, sacituzumab govitecan; IgG, immunoglobulin G; Dato-DXd, datopotamab deruxtecan; GGFG, Gly-Gly-Phe-Gly; pH, pondus hydrogenii.
Humanized TROP2 monoclonal antibody was selected for all three ADCs. However, the monoclonal antibody utilized in Dato-DXd was originally derived from mice immunized with NCI-H322 human lung adenocarcinoma cells (37), distinguishing it from the other two ADCs, whose antibodies were originally sourced from mice immunized with human squamous-cell carcinoma of the lung (39). TL033 (SKB264) is a derivative of CL2A (SG), both of which are pH-sensitive, while Dato-DXd uses GGFG tetrapeptide, which is sensitive to enzyme digestion, as a linker. Different nature of linkers can affect the release of drug load. When the ADCs were mixed and co-bathed with plasma respectively, the release rate of SG drug load reached 100% at 48 hours and 70% at 144 hours for SKB264 (38), whereas the 21-day drug load release rate for Dato-DXd did not exceed 6% (37), Dato-DXd is more stable in plasma than the other two ADCs. The drug load of the three ADCs includes all derivatives of camptothecin, a natural inhibitor of topoisomerase I (TOP 1). These three ADCs present “bystander effects” but with differences in cytotoxicity. As the DXd drug being the most cytotoxic, it is set at a DAR of 4:1 to reduce systemic toxicity. In contrast, SG and SKB264 chose a drug load with a cytotoxicity weaker than that of DXd and set the DAR close to 8:1 without affecting the body’s tolerance.
Based on the difference of the design concept and realization route of the three ADCs, the pharmacokinetic, pharmacodynamic, and adverse reaction characteristics also differ. For instance, SG features a pH-sensitive linker designed to release its drug payload not only in the classical intracellular acidic environment, but also in the tumor extracellular acidic environment before internalization (24). The ability to release the drug load both intracellularly and/or extracellularly, allows the drug load to penetrate and exert a cytotoxic effect on neighboring TROP2-low-expressing cancer cells, leading to a “double bystander effect”. The Dato-DXd uses an enzyme-sensitive linker, and its terminal elimination half-life (T1/2) is as long as 45.12 hours on average (37), at a dose of 6 mg/kg in cynomolgus monkeys. When injected into mice at 3 mg/kg, the T1/2 for SKB264 and SG were 56.3 and 15.5 hours, respectively (38). A deeper understanding of the clinical differences among the three ADCs will emerge as a new research topic.
Single-agent clinical trials of ADCs targeting TROP2
In the phase III ASCENT trial, SG was used to treat refractory or relapsed aTNBC. In total, 468 patients who had received ≥2 lines of chemotherapy (median four lines) and had no brain metastasis were randomly divided into two groups, with the control group receiving the standard treatment of the physician’s choice (TPC). The results reported that the median PFS of the SG and the TPC groups were 5.6 [95% confidence interval (CI): 4.3–6.3] and 1.7 months (95% CI: 1.5–2.6), respectively [hazard ratio (HR), 0.39; 95% CI: 0.14–1.07; P<0.001]. The median OS was 12.1 (95% CI: 10.7–14.0) and 6.7 months (95% CI: 5.8–7.7), respectively (HR, 0.48; 95% CI: 0.38–0.59; P<0.001). The ORR of the SG group was seven times that of the TPC group [35% (82/235) vs. 5% (11/233)]. Grade ≥3 TRAEs were mainly hematologic toxicity, such as neutropenia and diarrhea, which were tolerated by most patients after drug reduction, drug interruption, and symptomatic treatment (25,26). The EVER-132-001 was a multicenter bridging trial that verified the efficacy and safety of SG in 80 Chinese patients with aTNBC who had received ≥2 lines of standard chemotherapy. Tumor size reduction of ≥30% was observed in 50% (40/80) of patients, and no association between TRAEs and the UGT1A1 polymorphism, a factor associated with efficacy and toxicity of irinotecan-based therapy, was observed in the trial (27).
Of note, regardless of baseline germline BRCA1/2 mutation status, ORR, PFS, and OS were significantly improved in the SG group compared with the TPC group, indicating that the germline BRCA1/2 mutation status did not affect the efficacy of SG (16). Moreover, in the case of high, medium, and low expression of TROP2, the SG group was significantly better than the TPC group in median PFS, median OS, and ORR, indicating that all TROP2 subgroups with various expression levels benefitted from SG treatment compared with the TPC group (16,40). The TROPiCS-02 study further confirmed that SG was significantly more effective than chemotherapy, even in the presence of low TROP2 expression levels in cancerous tissue. This point suggests that the SG benefit was independent of TROP2 expression levels (41,42). It may be attributed to SG’s capacity to exert a “double bystander effect”, which enhances the cytotoxicity on cancer cells of the drug-loaded SN-38 (41).
The phase I TROPION-PanTumor01 trial enrolled 44 patients with mTNBC who received median three-line treatment. The ORR after Dato-DXd treatment was 32% (14/44), the disease control rate (DCR) was 80% (35/44), the median PFS was 4.3 months (95% CI: 3.0–7.3), the median OS was 12.9 months (95% CI: 10.1–14.7), and the most common grade ≥3 TRAEs were stomatitis, nausea, vomiting, and fatigue (28).
Study NCT04152499 was a phase II cohort extension non-randomized study that included 59 treated patients with mTNBC; of whom, 52 patients (88%) had received ≥3 lines of therapy, with an ORR of 40% (22/55) and DCR of 80% (44/55) after SKB264 treatment, and an incidence of grade ≥3 TRAEs of 55.9%. The most common AEs were neutropenia, anemia, and thrombocytopenia, with no TRAE-related deaths or interstitial lung disease (29). SKB264 has submitted an application for indication approval in China for the treatment of locally advanced or mTNBC in patients who have received at least two lines of therapy.
Single-agent clinical trials of ADCs targeting HER2
The HER2 regulates cell division and angiogenesis through multiple signaling pathways. The positive rate of HER2 in breast cancer is 15–25%, and it is associated with lymph node metastasis (43). The classification of HER2 status is based on superexpression of HER2 as determined by immunohistochemistry (IHC) and/or amplification of the ERBB2 by in situ hybridization (ISH). HER2+, corresponding to tumors eligible for HER2-target therapies, defined by strong and complete membrane staining in more than 10% of the tumor cells (score 3+), or complete weak/moderate staining in more than 10% of tumor cells (score 2+) and ISH non-amplified (44). HER2-negative (HER2−) include tumors without any membrane staining (score 0), tumors with incomplete and faint/barely membrane staining in up to 10% of tumor cells (score 0, HER2-ultra-low), tumors with incomplete and faint/barely perceptible in membrane staining in up to 10% of tumor cells (score 1+, HER2-low), and tumors with score 2+/ISH non-amplified (44,45). HER2− tumors can be HR+ (80.8%) or triple-negative (19.2%). Although the fraction of HER2-low is higher in HR+ tumors (65.4%) than in TNBC (36.6%), it is still relevant in this last group (46). However, HER2 expression has considerable heterogeneity, which specifically refers to the difference in HER2 gene expression status in the same region or different regions in a single tumor, including intra- and inter-tumor heterogeneity manifested by different molecular subtypes, mutation spectrum, HER2 protein expression level, and immunocyte invasion (47). The down-regulated HER2 expression of cancer cells, may have a significant weakening effect on the efficacy of ADCs targeting HER2 without the drug load of membrane permeability (e.g., trastuzumab emtansine), but have much less impact on trastuzumab deruxtecan (T-DXd) (48).
A relatively well-studied HER2-targeting ADC associated with TNBC is T-DXd, which is a HER2 monoclonal antibody linked to DXd via a cleavable tetrapeptide linker (49). The DESTINY-Breast04 study enrolled 557 patients with advanced breast cancer of HER2-low who had received a median of three lines of prior therapy. However, only 58 patients in the HR-/HER2-low subgroup were included. Owing to the limited number of cases in this subgroup and the fact that it was not a pre-specified subgroup to be analyzed, the efficacy of this subgroup could only be included as an exploratory endpoint. Results of improvements in PFS [8.5 (95% CI: 4.3–11.7) vs. 2.9 months (95% CI: 1.4–5.1)], OS [18.2 (95% CI: 13.6–not evaluable) vs. 8.3 months (95% CI: 5.6–20.6)], and ORR [50% (20/40) vs. 16.7% (3/18)] were observed in this subgroup treated with T-DXd compared with the TPC control group, while 16.2% of total patients in the study discontinued because of TRAEs (50). The DAISY study is a multicenter, phase II clinical trial, and the enrolled patients with metastatic breast cancer are divided into three cohorts: HER2+ (72 cases), HER2-low (74 cases), and HER2-zero (40 cases). The HER2-low cohort and HER2-zero cohort were treated with anthracyclines and taxanes, with some patients meeting the diagnostic criteria for TNBC. The results of the T-DXd treatment in the two cohorts reported that the ORR reached 37.5% (27/72) and 29.7% (11/37), with a median PFS of 6.7 (95% CI: 4.4–8.3) and 4.2 (95% CI: 2.0–5.7) months, respectively (30). The National Comprehensive Cancer Network (NCCN) guideline and the Chinese Society of Clinical Oncology (CSCO) guideline recommend T-DXd as the second-line treatment for patients with HER2-low (51,52). The China Anti-Cancer Association (CACA) guideline for the diagnosis and treatment of breast cancer (2024 abridged edition) indicates that T-DXd could be selected for the second-line treatment in HER2-low, even though it states that the evidence is based on a small sample size (53).
Single-agent clinical trials of ADCs targeting other antigens
In addition to TROP2 and HER2 antigens, other antigens such as HER3, B7-H4, and LIV-1 are currently relevant for ADC drug development in TNBC. HER2 can form heterodimers with other members of the HER family, of which the HER2/HER3 heterodimer has the strongest cell proliferation and growth-promoting signals, and HER3 overexpression is present in 20% to 30% of breast cancer patients (47). The B7 family of immune checkpoints plays a significant role in modulating immune cell functions, with B7-H4 being a transmembrane protein expressed in various tumors, including TNBC (54,55). As a zinc transporter transmembrane protein, LIV-1 is also expressed by TNBC cells (56). Table 4 presents preliminary clinical investigations with reported results for these antigens.
Table 4
| Primary information | U31402-A-J10 1 (57) | NCT05263479 (58) | NCT01969643 (59) |
|---|---|---|---|
| Study drug | HER3-DXd | HS-20089 | Ladiratuzumab vedotin |
| Target antigen | HER3 | B7-H4 | LIV-1 |
| Clinical staging | Phase I/II | Phase I | Phase I |
| Sample size (cases) | 53 | 16 | 40 |
| ORR (%) | 22.6 | 37.5 | 28.0 |
| Median PFS (months) | 5.5 | – | – |
| Median OS (months) | 14.6 | – | – |
| The most common TRAEs | Gastrointestinal and hematologic toxicities | Hematologic toxicities, gastrointestinal symptoms, and hepatic impairment | Gastrointestinal symptoms, fatigue, and peripheral sensory neuropathy |
–, no data. ADCs, antibody-drug conjugates; aTNBC, advanced triple-negative breast cancer; HER3, human epidermal growth factor receptor 3; DXd, deruxtecan; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; TRAEs, treatment-related adverse events.
Bispecific antibodies, a class of engineered antibodies that can simultaneously bind to two distinct antigens or receptors, are designed to redirect immune cells or deliver drug-load to specific cells or tissues (60). Preliminary exploration has also been carried out on bispecific ADC drugs for breast cancer. The prolactin receptor (PRLR) plays a significant role in certain breast cancers. Low levels of PRLR (approximately 3×104 receptors per cell) were sufficient for effective killing by a PRLR ADC, but higher levels of HER2 (approximately 106 receptors per cell) were required for cell killing by trastuzumab emtansine (60). In cancer cells where HER2 and PRLR were coexpressed, a HER2 × PRLR bispecific ADC exhibited superior cell-killing activity compared to a HER2-specific ADC (60). These results suggest bispecific ADCs may enhance the therapeutic effectiveness of ADC-based treatments in breast cancer.
Updated therapy for aTNBC recommended in guidelines
A comprehensive network meta-analysis was conducted to investigate the optimal treatment sequence for mTNBC. The analysis included 33 clinical studies involving 11,321 patients who had received at least second-line therapy. The results reported that SG was superior to other monotherapies on all study endpoints, as calculated by the surface under the cumulative ranking (SUCRA). In patients with HER2-low mTNBC, T-DXd was similar to SG in efficacy, but the improvement in PFS and OS was inferior to SG, suggesting that SG may be the optimal monotherapy for aTNBC (61).
On the basis of many convincing clinical studies, SG is unanimously recommended by various guidelines [e.g., NCCN, CSCO, CACA, and the European Society for Medical Oncology (ESMO)] as the preferred second-line treatment for aTNBC (51,52,62,63). Furthermore, both the ESMO-Magnitude of Clinical Benefit Scale online guideline and the ESMO consensus on HER2-low clearly recommend that SG should be selected before T-DXd for the relevant patients (45,64). To date, the clinical treatment patterns of aTNBC have entered a new era. For instance, the algorithm principles against aTNBC according to the Chinese guidelines are shown in Table 5.
Table 5
| Population | Treatment options |
|---|---|
| First line | |
| Biomarker-negative/unknown | Anthracycline/taxanes/platinum-based single-agent or combination chemotherapy regimens |
| Biomarker-positive | • Chemotherapy regimens ± PD-L1 inhibitors for PD-L1 positivity* |
| • PARP inhibitors or platinum-based chemotherapy regimens for gBRCA mutation* | |
| Later lines | |
| Biomarker-based | • Sacituzumab govitecan targeting TROP2# |
| • T-DXd targeting HER2-low | |
| General population | Based on prior drugs, length of progression-free interval, and disease status, treatment options may include eribulin, utidelone, and capecitabine, etc. |
*, indications for PD-L1 inhibitors and PARP inhibitors approved in China do not include breast cancer; #, routine testing of TROP2 levels is not mandatory currently. aTNBC, advanced triple-negative breast cancer; PD-L1, programmed cell death protein-ligand 1; PARP, poly (ADP-ribose) polymerase; gBRCA, germline breast cancer susceptibility gene; TROP2, trophoblast cell-surface antigen 2; T-Dxd, trastuzumab deruxtecan; HER2, human epidermal growth factor receptor 2.
Research progress on resistance to ADCs in aTNBC
The efficacy of SG in refractory aTNBC
Recurrence within 12 months after the completion of (neo)adjuvant therapy suggests that TNBC may be resistant to cytotoxic chemotherapy (65,66) or refractory (11). The ASCENT trial included 65 patients who experienced disease recurrence within 12 months after (neo)adjuvant chemotherapy and had received first-line therapy prior to enrolment. The efficacy and safety profile of SG for all key endpoints in this subgroup was consistent with those observed in the ASCENT intention-to-treat population, indicating that SG remains efficacious in patients who may be refractory to chemotherapy (12). Nevertheless, to minimize the adverse effects of cross-resistance on patients, it should be taken seriously that current guidelines recommend the preferred use of SG as a second-line regimen. This is to ensure that there is no excessive delay in initiating SG therapy.
Exploration of T-DXd resistance
The DAISY study also explored multiple factors that may influence the drug resistance, such as the level of HER2 expression, HER2 spatial distribution, ERBB2 gene expression, T-DXd tumor tissue distribution and SLX4 gene mutation (30). The results show that HER2 expression is a determinant of T-DXd efficacy, and that reduced HER2 expression is associated with drug resistance (30). In addition, the spatial distribution of HER2-ultra-low or the distribution of T-DXd in HER2-zero also affects resistance to T-DXd, but any efficacy difference based on ERBB2 gene expression within the group of patients with HER2-zero was not observed (30). Notably, T-DXd was still distributed in the cancer cells in some patients at the time of resistance, but TOP1 gene mutations was not detected. On the other hand, SLX4 gene, encoding a DNA repair protein to regulate structure-specific endonucleases and may have a role in resistance to TOP1 inhibition, was identified mutations at resistance in 14% (3/21) patients, even one patient was from the HER2+ cohort (30). These results suggest that resistance to T-DXd in cancer cells involves multiple mechanisms. Therefore, there is a need to look beyond the simple identification of HER2 immunostaining and explore additional biomarkers, such as quantitative continuous scoring of HER2, gene expression levels, and intrinsic drug sensitivity, to more comprehensively understand and predict the efficacy and resistance to T-DXd.
Exploratory study on sequential of ADCs single-agent
The ADC monotherapy may also lead to resistance similar to chemotherapy. Changes in the expression levels of molecules in cancer cells, including antigenic targets and/or drug-load targets, may mediate resistance to ADCs (67) and impact the sequential order of ADCs. The A3 real-world study reported that the overall PFS in HER2− breast cancer or TNBC treated with multiple ADCs was significantly reduced from 161 days for the first ADC to 77 days for the second ADC. The highest incidence of resistance was observed when both ADCs had the same target antigen with the same drug load, but it decreased if the antibody and/or drug load changed (68). The findings of this study indicate that the prior-line ADC therapy confers a superior survival benefit compared with subsequent ADCs. Additionally, the study suggests that sequential therapy with ADCs that have the same antigenic targets or drug-loaded targets should be avoided whenever possible. Two real-world studies in the United States (69) and France (70) compared the sequential efficacy of SG and T-DXd, with similar results to Study A3. HR expression levels or sequential order did not significantly affect aforementioned results (69), and delayed initiation of the subsequent ADCs may improve PFS compared with immediate initiation (70).
Owing to the overlapping indications of SG and T-DXd, for a comprehensive comparison and consideration, the ESMO consensus recommends that after the failure of the first-line chemotherapy for HER2-low advanced breast cancer, HR+ should give priority to T-DXd, and HR− (i.e., TNBC) should first consider SG (45). With the introduction of new ADCs, the optimization of ADC treatment sequences has become more interesting. Future prospective studies are needed to further assess the efficacy and safety of sequential ADCs and also investigate biomarkers or drug resistance in cancer responses.
Other research progress of ADCs in aTNBC
The combination of ADCs with immunotherapy
At present, the study of ADC combined with immunotherapy is in the exploratory stage. The randomized trial MORPHEUS-panBC preliminarily revealed that in patients with no prior systemic treatment for PD-L1-positive and inoperable aTNBC, compared with the atezolizumab and nab-paclitaxel combination, the atezolizumab and SG combination demonstrated the trends toward higher ORR and CBR, as well as a longer median PFS (71). The arm 6 (72) and arm 7 (73) of the BEGONIA study preliminarily reported that T-DXd or Dato-DXd in combination with durvalumab may improve the clinical benefit of patients with aTNBC. Preliminary indications from the SGNLVA-002 study suggest that ladiratuzumab vedotin combined with pembrolizumab may be effective against aTNBC (74). Other studies related to ADCs combined with immunotherapy are currently underway. The ASPRIA trial aims to investigate the efficacy and safety of SG in combination with atezolizumab for the prevention of TNBC recurrence (75). The InCITe trial is a randomized trial that will investigate the efficacy and safety of SG combined with avelumab for aTNBC (76). The Saci-IO trial will investigate the efficacy of SG in combination with pembrolizumab for PD-L1-negative aTNBC (77). The TROPION-Breast05 trial will compare the efficacy and safety of Dato-DXd ± durvalumab versus TPC combined with pembrolizumab in patients with PD-L1+ aTNBC (78).
The combination of ADCs with other drugs
The SEASTAR study initially investigated the efficacy of SG combined with the PARP inhibitor rucaparib, which resulted in all three cases that had progressed following PARP inhibitor treatment achieved a partial response, suggesting that SG in combination with PARP inhibitors may enhance efficacy against solid tumors, especially in carriers of the ataxia telangiectasia-mutated (ATM) gene (79). Study NCT05633979 will investigate the efficacy and safety of T-DXd combined with the enhancer of zeste homolog (EZH) 1/2 inhibitor valemetostat in the treatment of HER2-low/-ultra-low/-null advanced breast cancer (80).
ADCs for first-line treatment
The first-line treatment efficacy of SG is also being investigated in patients with aTNBC. The ASCENT-03 (81) and the ASCENT-04 (82) trials both enroll some untreated patients with aTNBC. The aim of the ASCENT-03 trial is to compare SG with TPC regimens, while the ASCENT-04 trial compares SG plus pembrolizumab with chemotherapy plus pembrolizumab for the first-line efficacy. The primary endpoint in both trials is PFS.
Prospects on research of ADCs for TNBC
First, ADC drugs in breast cancer shows a trend toward breaking the barrier of traditional molecular markers. The phase III TROPiCS-02 study included patients with HR+ or HER2− advanced breast cancer who had received at least two lines of chemotherapy. The results of this study presented that, in comparison to chemotherapy, SG significantly improved PFS, OS, ORR, health status, and quality of life (41,83,84). The phase III ASCENT-07 study will compare SG to TPC regimens in patients with HR+/HER2− (including HER2-low/-zero), inoperable or endocrine-treated metastatic breast cancer (85). Therefore, SG is breaking into HR+ breast cancer beyond the traditional TNBC. On the other hand, the phase III DESTINY-Breast15 study will investigate T-DXd in patients with HER2-low/-zero advanced breast cancer, with the primary endpoint being the time to next therapy or death (TTNTD) from the date of the T-DXd first initiation (86), which signifies that T-DXd is attempting to fully enter the TNBC therapeutic arena.
Second, the significance of the “bystander effects” of ADCs in targeted anticancer therapy needs to be further clarified in both SG targeting TROP2 and T-DXd targeting HER2. This phenomenon may have particular clinical implications for overcoming resistance mechanisms resulting from a decrease in antigen molecular expression. The investigation of more effectively utilizing the “bystander effect” may become an important research direction for developing high-efficiency and low-toxicity anti-cancer drugs in the future.
Third, the optimization of the use of ADCs and the investigation of cancer resistance mechanisms to ADCs are yet to be performed. Compared with traditional chemotherapy, the resistance mechanism to ADCs is complex and diverse, which has not been fully revealed so far (49), and there may be a cross-resistance phenomenon among ADCs (68). In this regard, real-world studies suggest that with the availability of multiple ADCs, there is a growing interest in the sequencing of ADCs. In addition, the investigation of the potential for combining certain ADCs with other anti-cancer drugs may represent a significant avenue for the future development of strategies to mitigate resistance and enhance the efficacy of ADCs. Concurrently, investigations on ADC treatment for operable patients with TNBC, when confirmatory evidence of efficacy is obtained, may help prevent, intervene, and control ADC resistance in the early stages of cancer.
Finally, the use of ADCs to treat aTNBC necessitates the incorporation of safety management as a fundamental aspect of the practice. Each ADC in TNBC has its own strengths and weaknesses in terms of safety. In the DESTINY-Breast04 trial, drug-related deaths included two pneumonia (0.5%) and one ischemic colitis, one disseminated intravascular coagulation, one dyspnea, one febrile neutropenia, and one sepsis each (0.3%) in the T-DXd group, compared with no drug-related deaths in the control group; the most common TRAEs included nausea (73.0%), fatigue (47.7%), and alopecia (37.7%), which were significantly higher than those in the control group. In addition, the incidence of drug-related grade ≥3 cardiac ejection impairment was 1.5% in the T-DXd group, compared with no TRAEs of this grade in the control group (50). The results from the ASCENT study reported that the incidence of major adverse events of grade ≥3 associated with SG treatment included neutropenia (51%), leukopenia (10%), diarrhea (10%), anemia (8%), and granulocytopenia fever (6%), with three deaths each in the SG and control groups, but the deaths were not related to the SG (25). As for the safety of SG, the FDA indicates that the adverse reactions are mainly neutropenia and diarrhea during the treatment. In the event that the above symptoms are of a serious nature, it is recommended that the treatment include the discontinuation or reduction of SG, infusion of colony stimulating factor, anti-infection, anti-diarrheal, rehydration, and other supportive treatments as appropriate (87). Compared with the management of pneumonia or cardiotoxicity, these therapeutic measures are easier to conduct. Owing to its remarkable efficacy, safety profile, and potential for combined therapy, SG may emerge as a pivotal component of future clinical TNBC treatment regimens (88). Corroborating this observation, the progress of SG in first-line therapy is increasingly emphasized in real clinical settings (89).
In the current review, only English and Chinese literature were selected during the literature search phase, and other languages were excluded, which may affect the inclusion of documents to some extent, thus selection bias cannot be avoided. Similarly, when reviewing the relevant literature, we selectively traced back to the cited references, which may also introduce bias. However, our strength lies in the comprehensive and in-depth analysis of the progress in the research of ADCs against aTNBC, including discussions on pharmacological mechanisms, resistance mechanisms, and rational drug use, which could benefit clinical practice and related research.
Conclusions
In summary, TNBC is the most challenging area in the treatment of breast cancer. The treatment strategy for aTNBC has long been based on chemotherapy, and the survival and quality of life of patients are shrouded in the shadow of cancer. Although chemotherapy-, ICI-, and PARP-targeted therapies have made some progress in recent years, they have not yet met the clinical needs. On the other hand, as Paul Ehrlich proposed the concept of “magic bullet” a century ago, after years of research and continuous innovation, ADCs have finally successfully entered the stage of history. This represents that an important step has been taken in achieving the treatment goal of prolonging patient survival time and improving the quality of life of patients, which may indicate the dawn of the aTNBC precision treatment. As the field of ADC drugs continues to evolve, it is anticipated that new breakthroughs will emerge in the treatment of aTNBC. These advances will likely result from the deepening of ADCs alone or in combination with other drugs, as well as the accumulation of experience. Finally, these advances are expected to bring greater benefits to patients.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-38/rc
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-38/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-38/coif). S.W. serves as an unpaid editorial board member of Translational Breast Cancer Research from March 2024 to February 2026. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guidelines for clinical diagnosis and treatment of advanced breast cancer in China (2022 edition). Zhonghua Zhong Liu Za Zhi 2022;44:1262-87. [PubMed]
- Xiao YL, Zhu XZ, Jiang YZ, et al. New research advances and future prospect in precision treatment of triple-negative breast cancer. China Oncology 2022;32:668-79.
- Chen JY, Zhang WT, Liu Q, et al. Clinical dilemma and systemic treatment strategy of triple-negative breast cancer in the elderly. China Oncology 2023;33:506-16.
- Song WW, Sun JL. Advances on the application of tumor stem cell therapy in the molecular typing and targeted therapy of triple-negative breast cancer. The Practice Journal of Cancer 2023;38:344-8.
- Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ 2023;381:e071674. [Crossref] [PubMed]
- Dri A, Arpino G, Bianchini G, et al. Breaking barriers in triple negative breast cancer (TNBC) - Unleashing the power of antibody-drug conjugates (ADCs). Cancer Treat Rev 2024;123:102672. [Crossref] [PubMed]
- Li CY, Zhang S, Zhang XB, et al. Clinicopathological and prognostic characteristics of triple- negative breast cancer (TNBC) in Chinese patients: a retrospective study. Asian Pac J Cancer Prev 2013;14:3779-84. [Crossref] [PubMed]
- Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 2007;109:1721-8. [Crossref] [PubMed]
- Van Mechelen M, Van Herck A, Punie K, et al. Behavior of metastatic breast cancer according to subtype. Breast Cancer Res Treat 2020;181:115-25. [Crossref] [PubMed]
- Jeon Y, Jo U, Hong J, et al. Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer 2022;22:1014. [Crossref] [PubMed]
- Jiang MP, Huang X, Yin YM, et al. The pathological and clinical landscape of refractory metastatic triple negative breast cancer: a narrative review. Ann Transl Med 2022;10:907. [Crossref] [PubMed]
- Carey LA, Loirat D, Punie K, et al. Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer-phase 3 ASCENT study subanalysis. NPJ Breast Cancer 2022;8:72. [Crossref] [PubMed]
- Saini KS, Punie K, Twelves C, et al. Antibody-drug conjugates, immune-checkpoint inhibitors, and their combination in breast cancer therapeutics. Expert Opin Biol Ther 2021;21:945-62. [Crossref] [PubMed]
- Winer EP, Lipatov O, Im SA, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:499-511. [Crossref] [PubMed]
- Morrison L, Okines A. Systemic Therapy for Metastatic Triple Negative Breast Cancer: Current Treatments and Future Directions. Cancers (Basel) 2023;15:3801. [Crossref] [PubMed]
- Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol 2021;32:1148-56. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;30:558-66. [Crossref] [PubMed]
- Litton JK, Hurvitz SA, Mina LA, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol 2020;31:1526-35. [Crossref] [PubMed]
- Professional Committee on Clinical Research of Oncology Drugs CA-CA, Expert Committee for Monitoring the Clinical Application of Antitumor Drugs, Breast Cancer Expert Committee of National Cancer Quality Control Center, et al. Zhonghua Zhong Liu Za Zhi 2023;45:741-62. [Expert consensus on the clinical application of antibody drug conjugates in the treatment of malignant tumors (2023 edition)]. [PubMed]
- Xu YX, Zhang L, Qiao XW, et al. Efficacy and safety of antibody-drug conjugates in the treatment of breast cancer: a meta-analysis. Zhongguo Yaofang 2023;34:2540-4.
- Fu Z, Li S, Han S, et al. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther 2022;7:93. [Crossref] [PubMed]
- Shastry M, Jacob S, Rugo HS, et al. Antibody-drug conjugates targeting TROP-2: Clinical development in metastatic breast cancer. Breast 2022;66:169-77. [Crossref] [PubMed]
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 2021;384:1529-41. [Crossref] [PubMed]
- Bardia A, Tolaney SM, Loirat D, et al. Sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (pts) with previously treated, metastatic triple-negative breast cancer (mTNBC): Final results from the phase 3 ASCENT study. J Clin Oncol 2022;40:1071. [Crossref]
- Xu B, Ma F, Wang T, et al. A Phase IIb, single arm, multicenter trial of sacituzumab govitecan in Chinese patients with metastatic triple-negative breast cancer who received at least two prior treatments. Int J Cancer 2023;152:2134-44. [Crossref] [PubMed]
- Bardia A, Krop I, Meric-Bernstam F, et al. Abstract P6-10-03: Datopotamab Deruxtecan (Dato-DXd) in Advanced Triple-Negative Breast Cancer (TNBC): Updated Results From the Phase 1 TROPION-PanTumor01 Study. Cancer Res 2023;83:P6-10-03.
- Yin Y, Wu X, Ouyang Q, et al. Abstract OT1-03-02: Efficacy and safety of SKB264 for previously treated metastatic triple negative breast cancer in Phase 2 study. Cancer Res 2023;83:OT1-03-2.
- Mosele F, Deluche E, Lusque A, et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat Med 2023;29:2110-20. [Crossref] [PubMed]
- Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015;6:22496-512. [Crossref] [PubMed]
- Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 2015;6:84-105. [Crossref] [PubMed]
- Lin Y, Zhang Y, Chen X. Advances in the treatment of triple-negative breast cancer with antibody-drug conjugates targeting Trop2. Chinese Journal of Clinical Oncology 2023;50:946-50.
- Zhang Z, Jia LZ, Tang Q, et al. Expressions of TROP2 and VEGFR2 and their relationship with clinico - pathological factors in triple negative breast cancer. J Nanjing Med Univ 2019;39:1453-8, 71.
- Bardia A, Mayer IA, Diamond JR, et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J Clin Oncol 2017;35:2141-8. [Crossref] [PubMed]
- Zhao W, Kuai X, Zhou X, et al. Trop2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol Rep 2018;40:759-66. [Crossref] [PubMed]
- Okajima D, Yasuda S, Maejima T, et al. Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol Cancer Ther 2021;20:2329-40. [Crossref] [PubMed]
- Cheng Y, Yuan X, Tian Q, et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front Oncol 2022;12:951589. [Crossref] [PubMed]
- Stein R, Basu A, Chen S, et al. Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int J Cancer 1993;55:938-46. [Crossref] [PubMed]
- Bartsch R. SABCS 2020: update on triple-negative and metastatic HER2-positive breast cancer. Memo 2021;14:247-51. [Crossref] [PubMed]
- Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet 2023;402:1423-33. [Crossref] [PubMed]
- Rugo H, Bardia A, Marmé F, et al. Abstract GS1-11: Sacituzumab Govitecan (SG) vs Treatment of Physician’s Choice (TPC): Efficacy by Trop-2 Expression in the TROPiCS-02 Study of Patients (Pts) With HR+/HER2– Metastatic Breast Cancer (mBC). Cancer Res 2023;83:GS1-11. [Crossref]
- The Professional Committee of Tumor Biomarker, Chinese Anti-cancer Association. The clinical practice expert consensus of tumor biomarkers in recurrent or metastatic breast cancer (2019). Chinese Journal of Oncology Prevention and Treatment 2019;11:363-74.
- Wolff AC, Somerfield MR, Dowsett M, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol 2023;41:3867-72. [Crossref] [PubMed]
- Tarantino P, Viale G, Press MF, et al. ESMO expert consensus statements (ECS) on the definition, diagnosis, and management of HER2-low breast cancer. Ann Oncol 2023;34:645-59. [Crossref] [PubMed]
- Schettini F, Chic N, Brasó-Maristany F, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021;7:1. [Crossref] [PubMed]
- The Expert Board of Breast Cancer Biomarkers, Tumor Marker Committee of Chinese Anti-cancer Association. Expert consensus on clinical application of biomarkers for precision treatment of breast cancer based on target guidance (2022 edition). Chinese Journal of Oncology Prevention and Treatment 2022;14:346-62.
- Giugliano F, Corti C, Tarantino P, et al. Bystander effect of antibody-drug conjugates: fact or fiction? Curr Oncol Rep 2022;24:809-17. [Crossref] [PubMed]
- Chen KY, Huang Y, Wang XJ, et al. Clinical application and research progress of antibody drugs conjugation in breast cancer. Chinese Journal of Clinical Pharmacology and Therapeutics 2023;28:898-909.
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Gradishar WJ, Moran MS, Abraham J, et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J Natl Compr Canc Netw 2023;21:594-608. [Crossref] [PubMed]
- Working group for Guidelines of Chinese Society of Clinical Oncology. Chinese Society of Clinical Oncology (CSCO): Guidelines of Breast Cancer 2023. Beijing: Peoples Health Publishing House; 2023.
- The Society of Breast Cancer China Anti-Cancer Association, Breast Oncology Group of the Oncology Branch of the Chinese Medical Association. Guidelines for clinical diagnosis and treatment of advanced triple negative breast cancer in China (2024 Abridged Edition). Shanghai: Fudan University Press; 2024.
- Pulanco MC, Madsen AT, Tanwar A, et al. Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies. Cell Mol Immunol 2023;20:694-713. [Crossref] [PubMed]
- Sanuki F, Mikami Y, Nishimura H, et al. Immunohistological analysis of B7-H4, IDO1, and PD-L1 expression and tumor immune microenvironment based on triple-negative breast cancer subtypes. Breast Cancer 2023;30:1041-53. [Crossref] [PubMed]
- Sussman D, Smith LM, Anderson ME, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther 2014;13:2991-3000. [Crossref] [PubMed]
- Krop IE, Masuda N, Mukohara T, et al. Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3-Directed Antibody-Drug Conjugate, in Patients With Previously Treated Human Epidermal Growth Factor Receptor 3-Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial. J Clin Oncol 2023;41:5550-60. [Crossref] [PubMed]
- Wu J, Zhang J, Li H, et al. 381O First-in-human/phase I trial of HS-20089, a B7-H4 ADC, in patients with advanced solid tumors. Ann Oncol 2023;34:S336. [Crossref]
- Tsai M, Han HS, Montero AJ, et al. 259P Weekly ladiratuzumab vedotin monotherapy for metastatic triple-negative breast cancer. Anna Oncol 2021;32:S474-5. [Crossref]
- Lan HR, Chen M, Yao SY, et al. Bispecific antibodies revolutionizing breast cancer treatment: a comprehensive overview. Front Immunol 2023;14:1266450. [Crossref] [PubMed]
- Schettini F, Venturini S, Giuliano M, et al. Multiple Bayesian network meta-analyses to establish therapeutic algorithms for metastatic triple negative breast cancer. Cancer Treat Rev 2022;111:102468. [Crossref] [PubMed]
- The Society of Breast Cancer China Anti-Cancer Association, Breast Oncology Group of the Oncology Branch of the Chinese Medical Association. Guidelines for breast cancer diagnosis and treatment by China Anti-cancer Association (2024 edition). China Oncology 2023;33:1092-186.
- Im SA, Gennari A, Park YH, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, staging and treatment of patients with metastatic breast cancer. ESMO Open 2023;8:101541. [Crossref] [PubMed]
- Curigliano G, Castelo-Branco L, Gennari A, et al. ESMO Metastatic Breast Cancer Living Guidelines | ESMO. 2023. Available online: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/triple-negative-breast-cancer
- Melvin JC, Purushotham AD, Garmo H, et al. Progression of breast cancer following locoregional ipsilateral recurrence: importance of interval time. Br J Cancer 2016;114:88-95. [Crossref] [PubMed]
- Yardley DA. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int J Breast Cancer 2013;2013:137414. [Crossref] [PubMed]
- Coates JT, Sun S, Leshchiner I, et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov 2021;11:2436-45. [Crossref] [PubMed]
- Abelman RO. PS-08-03 Sequencing Antibody-Drug Conjugate after Antibody-Drug Conjugate in Metastatic Breast Cancer (A3 study): Multi-Institution Experience and Biomarker Analysis.: SABCS; 2023.
- Huppert LA, Mahtani R, Fisch S, et al. PS08-04 Multicenter retrospective cohort study of the sequential use of the antibody-drug conjugates (ADCs) trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG) in patients with HER2-low metastatic breast cancer (MBC). SABCS; 2023.
- Poumeaud F. PS-08-02 Efficacy of Sacituzumab Govitecan (SG) post Trastuzumab Deruxtecan (T-DXd) and vice versa for HER2-low advanced or metastatic breast cancer (MBC): a French multicentre retrospective study.: SABCS; 2023.
- Schmid P, Loi S, De la Cruz Merino L, et al. 181O Interim analysis (IA) of the atezolizumab (atezo) + sacituzumab govitecan (SG) arm in patients (pts) with triple-negative breast cancer (TNBC) in MORPHEUS-pan BC: A phase Ib/II study of multiple treatment (tx) combinations in pts with locally advanced/metastatic BC (LA/mBC). ESMO Open 2024;9:103203. [Crossref]
- Schmid P. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J Clin Oncol 2021;39:1023. [Crossref]
- Schmid P, Jung KH, Wysocki PJ, et al. 166MO Datopotamab deruxtecan (Dato-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic triple-negative breast cancer (a/mTNBC): Initial results from BEGONIA, a phase Ib/II study. Ann Oncol 2022;33:S199. [Crossref]
- Subhan MA, Torchilin VP. Advances in Targeted Therapy of Breast Cancer with Antibody-Drug Conjugate. Pharmaceutics 2023;15:1242. [Crossref] [PubMed]
- Dana-Farber Cancer Institute, Genentech Inc, Stand Up To Cancer. Atezolizumab + Sacituzumab Govitecan to Prevent Recurrence in TNBC (ASPRIA). 2025. Available online: https://classic.clinicaltrials.gov/show/NCT04434040
- Hope Rugo, Translational Breast Cancer Research C, Hoosier Cancer Research Network, et al. Avelumab With Binimetinib, Sacituzumab Govitecan, or Liposomal Doxorubicin in Treating Stage IV or Unresectable, Recurrent Triple Negative Breast Cancer. 2025. Available online: https://classic.clinicaltrials.gov/show/NCT03971409
- Garrido-Castro AC, Keenan TE, Li T, et al. Saci-IO TNBC: Randomized phase II trial of sacituzumab govitecan (SG) +/- pembrolizumab in PD-L1– metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2021;39:TPS1106. [Crossref]
- AstraZeneca, Daiichi Sankyo I. A Phase III Study of Dato-DXd With or Without Durvalumab Compared With Investigator's Choice of Chemotherapy in Combination With Pembrolizumab in Patients With PD-L1 Positive Locally Recurrent Inoperable or Metastatic Triple-negative Breast Cancer. 2026. Available online: https://classic.clinicaltrials.gov/show/NCT06103864
- Yap TA, Hamilton E, Bauer T, et al. Phase Ib SEASTAR Study: Combining Rucaparib and Sacituzumab Govitecan in Patients With Cancer With or Without Mutations in Homologous Recombination Repair Genes. JCO Precis Oncol 2022;6:e2100456. [Crossref] [PubMed]
- M. D. Anderson Cancer Center, Daiichi Sankyo I. Phase 1b Study of EZH1/2 Inhibitor Valemetostat in Combination With Trastuzumab Deruxtecan in Subjects With HER2 Low/Ultra-low/Null Metastatic Breast Cancer. 2030. Available online: https://classic.clinicaltrials.gov/show/NCT05633979
- Gilead Sciences. Study of Sacituzumab Govitecan-hziy Versus Treatment of Physician's Choice in Patients With Previously Untreated Locally Advanced Inoperable or Metastatic Triple-Negative Breast Cancer. 2027. Available online: https://classic.clinicaltrials.gov/show/NCT05382299
- Gilead Sciences, Merck Sharp, Dohme L. L. C. Study of Sacituzumab Govitecan-hziy and Pembrolizumab Versus Treatment of Physician's Choice and Pembrolizumab in Patients With Previously Untreated, Locally Advanced Inoperable or Metastatic Triple-Negative Breast Cancer. 2027. Available online: https://classic.clinicaltrials.gov/show/NCT05382286
- Marmé F, Bardia A, Rugo H, et al. Abstract P4-07-65: Effect of sacituzumab govitecan vs chemotherapy in HR+/HER2- metastatic breast cancer: patient-reported outcomes from the TROPiCS-02 trial. Cancer Res 2023;83:P4-07-65.
- Rugo HS, Bardia A, Marmé F, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol 2022;40:3365-76. [Crossref] [PubMed]
- Gilead Sciences. Study of Sacituzumab Govitecan Versus Treatment of Physician's Choice in Patients With Hormone Receptor-positive/Human Epidermal Growth Factor Receptor 2 Negative (HR+/HER2-) Metastatic Breast Cancer Who Have Received Endocrine Therapy. 2025. Available online: https://classic.clinicaltrials.gov/show/NCT05840211
- Daiichi Sankyo I, AstraZeneca. Trastuzumab Deruxtecan (T-DXd) in Patients Who Have Hormone Receptor-negative and Hormone Receptor-positive HER2-low or HER2 IHC 0 Metastatic Breast Cancer. 2027. Available online: https://classic.clinicaltrials.gov/show/NCT05950945
- Food and Drug Administration. FDA approves sacituzumab govitecan-hziy for HR-positive breast cancer. FDA 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sacituzumab-govitecan-hziy-hr-positive-breast-cancer
- Kalinsky K, Diamond JR, Vahdat LT, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol 2020;31:1709-18. [Crossref] [PubMed]
- Kalinsky K, Spring L, Yam C, et al. Real-world outcomes in patients (pts) with metastatic triple-negative breast cancer (mTNBC) treated with sacituzumab govitecan (SG) in 2L+ in the United States (US). J Clin Oncol 2023;41:e18879. [Crossref]
Cite this article as: Jiang K, Wang S. Advances in antibody-drug conjugates in the treatment of advanced triple-negative breast cancer: a narrative review. Transl Breast Cancer Res 2025;6:9.


