
Neurological symptom management in breast cancer meningeal carcinomatosis
Introduction
Treatment of brain metastases in solid cancers exerts a certain therapeutic effect with the advent of stereotactic radiotherapy, an excellent local therapy. At present, it is further improving with the advent of molecular targeted drug therapy, an excellent systemic therapy.
Among brain metastases, the pathological condition in which cancer cells that are targeted by stereotactic radiotherapy spread to the cerebrospinal fluid (CSF) cavity is called meningeal carcinomatosis (MC). MC is the most troublesome and intractable complication of central nervous system complications of solid cancers.
MC is common in breast cancer, clinically seen in approximately 5% of cases, and is considered common in invasive lobular carcinoma (1). Patients with MC present to oncologists with various complaints, including meningeal signs such as headache, vomiting, and neck stiffness, mild symptoms such as diplopia, hearing loss, hydrocephalus-related cognitive impairment, severe headache, and sciatica, and unconsciousness in severe cases. These symptoms are caused by multiple pathologic states, which can lead to diagnosis and treatment delays in clinical practice (2).
In lung cancer, advances in molecular targeted drug therapy have resulted in the selection of systemic therapy for MC, however, this is limited to suitable patients. In breast cancer, tyrosine kinase inhibitors (TKIs) and antibody drug conjugates (ADCs) have also been reported to be effective in some cases (3), however, their use remains limited. Given the lack of established standard treatments for MC, the treatment is focused on symptom relief.
The appropriate diagnosis and treatment of MC requires an understanding of the pathological states that present diverse symptoms. This review discusses the treatment of MC in breast cancer, particularly from the perspective of neurological symptom relief, based on author’s experience in clinical practice and literature review.
Characteristics of MCs in breast cancer
Brain metastasis is observed in 10% of patients diagnosed with advanced non-small cell lung cancer (NSCLC). Conversely, in metastatic breast cancer, brain metastasis occurs in 40% of patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer, 30% with triple-negative (TN) metastatic breast cancer, and 15% of hormone receptor (HR)-positive breast cancer (4). Among metastatic brain tumors, the most common primary site is lung cancer, accounting for 50% of all cases, followed by breast cancer with approximately 15% (5).
Epidermal growth factor receptor (EGFR)-mutant NSCLC cases are several times more likely to have MC than nonmutant cases (6). Moreover, hormone status and HER2 status determine the condition in breast cancer, with TN and HER2-positive cases particularly likely to have MC (7).
In brain metastasis from breast cancer, metastasis to the cerebrum is also common in cases with lung metastasis. However, brain metastasis to the posterior fossa via the Batson vertebral venous plexus is often seen in bone metastases such as to the vertebrae (8). These two pathways are possibly associated with MC development, and pathways involving direct invasion from vertebral and intracranial and spinal dura matter metastases are also thought to be more likely in breast cancer (9,10).
In addition, because trastuzumab is effective in HER2-positive cases of breast cancer, the number of MC cases is speculated increase because of prolonged survival.
Furthermore, TKIs are deemed effective in mutant cases in MC of lung cancer (11), whereas ADCs such as trastuzumab deruxtecan (T-DXd) and TKIs such as tucatinib are effective in breast cancer (12,13).
Five pathological conditions of MCs
MC exhibits various symptoms and signs. For ease of understanding, Table 1 shows the five pathological conditions in MC, cancer cells location, typical symptoms and critical signs.
Table 1
| Pathological condition | Meningitis | Hydrocephalus (ICH) | Focal brain damage | Spinal cord damage | Cranial nerve disorder |
|---|---|---|---|---|---|
| Cancer location | Meninges | CSF space | Parenchymal | Spinal pia & dura | Cranial nerve |
| Symptom & sign | Headache/nausea | Focal brain sign | Sciatica | Double vision | |
| Back pain | Blurred vision | Gait disturbance | Deafness | ||
| Critical sign | Stiff neck | Loss of consciousness | Epilepsy | Spinal conus syndrome/cauda equina syndrome | Garcin syndrome |
MC, meningeal carcinomatosis; ICH, intracranial hypertension; CSF, cerebrospinal fluid.
Meningitis
Meningitis symptoms and signs are caused by leptomeningeal inflammation due to cancer cells that have invaded the CSF cavity. The classic triad of symptoms is headache, vomiting, and neck stiffness, however, the disease is often discovered late, and patients may complain of mild appetite loss, persistent headaches, or pain behind the eyes. Then, patients begin to complain of back pain, and the critical sign of a progressive condition is a stiff neck.
Hydrocephalus-related intracranial hypertension
Intracranial hypertension can be considered a symptom of hydrocephalus, in which cancer cells accumulate in the arachnoid granulations, which are the site of CSF absorption, and prevent CSF absorption. The classic triad of symptoms is headache, vomiting, and blurred vision due to a choked disc. In mild cases, it may be diagnosed based on complaints of morning headaches or inability to eat despite not having undergone chemotherapy. If these symptoms arise, they will persist without remission, resulting in severe headaches, and progressing to mental signs or loss of consciousness.
The condition of focal brain damages
Focal brain damage to the cerebrum, cerebellum, and brainstem is a sign of coexisting metastasis to the brain parenchyma. Epilepsy is classified as a severe form. Naturally, epilepsy is thought to be caused by a combination of epileptogenic factors, however, for ease of understanding, it is considered herein as a single condition.
Spinal cord damage
Symptoms of spinal cord damage are caused by the accumulation of cancer cells in the spinal cavity causing pathological damage to the pia and dura mater of the spinal cavity. Symptoms include lower limbs numbness, walking difficulties, and other conditions. Severe forms include spinal conus syndrome and cauda equina syndrome. In breast cancer, bone metastasis to the spine is common, and infiltration of the spinal dura and direct infiltration into CSF cavity is also observed. It is caused by spinal inflammation of the surface of the spinal cord and hypertrophic inflammation of the dura mater, causing neuropathic pain corresponding to the location.
Cranial nerve disorders
The cranial nerves are exposed to the CSF from the CSF cavity until they pass through the skull, making them prone to injury. Injuries to the vestibulocochlear, trigeminal, facial, and ocular motor nerves may present with symptoms such as hearing loss, facial numbness, facial distortion, and diplopia. In severe cases, Garcin syndrome, a unilateral multiple cranial nerve disorder may occur (14). Multiple cranial nerve disorders can cause the deterioration in the performance of activities of daily living.
Anatomically, these encompass all symptoms and signs. These five pathological conditions can occur alone or in combination. Diverse symptoms and signs can occur at various times, which can lead to diagnostic difficulties and delayed treatment for MC.
Diagnosis
In clinical practice, MC is confirmed by CSF examination and neuroimaging. Although CSF examination is invasive and unfamiliar to an oncologist, it is not a difficult procedure. Physicians who are unfamiliar with neurological symptoms tend to contraindicate lumbar puncture in cases of increased intracranial pressure. However, in MC, increased intracranial pressure occurs gradually and in stages, thus CSF collection must be performed slowly when draining it, because this will not induce brain herniation. Above all, nausea may subside with one CSF drainage. In addition, because no changes may be observed in neuroimaging in the early stages, if there is any doubt, a CSF examination is recommended, particularly in mild cases.
CSF examination
The definitive diagnosis is made by CSF cytology. However, if general examinations showed a lymphocyte-dominant increase in cells and an elevation in the CSF protein concentration, which are suspected findings of meningitis, the diagnosis is quite certain. In addition, high protein concentrations may be a sign of intraspinal cavity lesion (15), and the CSF will appear yellowish. In some cases, the Froin’s sign in CSF may be positive, and the protein may form clumps (16,17).
Neuroimaging
Neuroimaging mainly involves contrast-enhanced head computed tomography (CT), or contrast-enhanced magnetic resonance imaging (MRI). MC has no specific CT or MRI findings, and the diagnosis is made based on findings of meningitis, hydrocephalus, abnormal cranial nerve enhancement, inflammation around the pituitary gland, inflammation around the pia matter of the spinal cord, and reaction of the spinal dura mater.
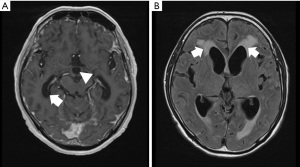
Meningitis findings include linear enhancements along the cerebellar folia and the brainstem. Figure 1 shows a T1-weighted image (T1WI) of a contrast-enhanced head MRI of a patient with MC. Figure 1A,1B shows the linear enhancement around the brainstem and cerebellar folia and along the cerebral sulci of the longitudinal fissure respectively.
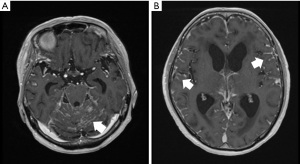
Early signs of hydrocephalus are often seen in images as a rounded form at the optic nerve crossing of the third ventricle. In addition, the inferior horn of the lateral ventricle is enlarged in the image (Figure 2A). When the entire lateral ventricle is enlarged, water infiltration into the brain parenchyma called the periventricular high-intensity area is also seen in fluid-attenuated inversion recovery (FLAIR) images on head MRI (Figure 2B).
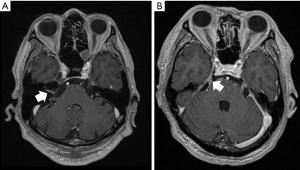
Abnormal cranial nerve enhancements are nearly never seen in the ocular motor nerves and are most seen in the vestibular or facial nerves in the internal auditory canal, followed by the trigeminal nerve. Other cranial nerves are extremely rarely observed in contrast imaging. Figure 3A,3B shows contrast enhancement in the right internal auditory canal, and the trigeminal nerve respectively.
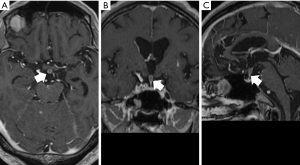
The pituitary stalk is also sometimes encountered as an inflammatory finding when present in the CSF cavity. Figure 4 shows axial (Figure 4A), coronal (Figure 4B), and sagittal (Figure 4C) images of the pituitary gland on contrast-enhanced MRI of the head. MRI demonstrates the thickened contrast enhancement around the pituitary stalk.
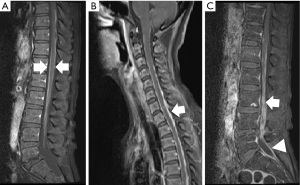
Spinal MRI shows linear contrast enhancement known as the railway sign, which is an inflammatory finding on the surface of the spinal cord (Figure 5A). In addition to these findings, abnormal contrast and dura mater thickening are observed, mainly at the lower end of the spinal dura mater. Furthermore, inflammatory findings on the surface of the cervical and thoracic spinal cord and thickening of some of the dura maters are observed in the sagittal section image of the contrasted MRI of the spinal cord (Figure 5B). In Figure 5C, abnormal contrast is observed in the inflamed area of the spinal cord and abnormal enhancement at the lower end of the spinal dura mater.
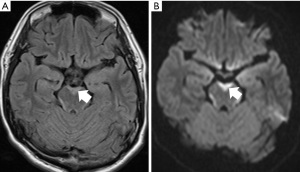
Although band-like hyperintensity is an exceptional finding, it may be seen around the midbrain in FLAIR images and diffusion-weighted images of head MRI in cases of EGFR-positive lung cancer (18,19). Figure 6 shows the FLAIR images and diffusion-weighted MRI of the head. In this case, this finding was seen as a high-intensity area surrounding the brain stem.
Treatment
The treatment algorithm in Figure 7 shows the current management of the neurological symptoms of MC, including reports, expert opinions, and personal experience.
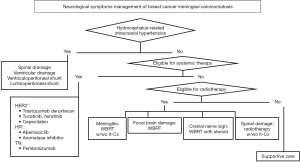
When MC is diagnosed, hydrocephalus must be treated immediately. This condition can progress rapidly, and if symptoms of increased intracranial pressure occur quickly, such as severe visual impairment and mental symptoms, lumbar drainage is necessary. This can be performed as a simple procedure in the hospital room under local anesthesia. The placement of an Ommaya reservoir in the ventricle, a ventriculoperitoneal shunt under general anesthesia, and a lumboperitoneal shunt under lumbar anesthesia are also possible (20,21).
The next consideration is whether systemic therapy is eligible or not. In NSCLC, TKIs are effective in treating EGFR mutations and should be used proactively to alleviate the symptoms of MC (22,23).
In breast cancer, drugs that are considered for MC include the tucatinib (a TKI), capecitabine (5-fluorouracil prodrug), T-DXd (an ADC), lapatinib (EGFR and HER2), and neratinib (a TKI) in the HER2-positive subtype group (24-28). In the HR-positive group, abemaciclib (the cyclin-dependent kinase 4/6 inhibitor) and hormone therapy have been tried (29). In the TN group, the immune checkpoint inhibitor PD-1 inhibitor pemprolizumab is expected to be a promising treatment (30).
Currently, if tucatinib or T-DXd is applicable, it will be the first-line treatment after MC diagnosis (31,32).
If the above systemic treatments are not applicable, the question arises as to whether radiation therapy can be used. If whole-brain radiation therapy (WBRT) has already been used or if the spine has been irradiated, radiation therapy is no longer indicated and supportive care is provided. In cases where radiation therapy is possible, a management method is selected according to the main MC symptoms. If meningitis symptoms are the main symptom, studies have reported that WBRT alone or in combination with intrathecal chemotherapy is effective. Several drugs have been reported for intrathecal administration, and methotrexate, thiotepa, topotecan, cytarabine, liposomal cytarabine, and trastuzumab are particularly promising (33-36). Despite concerns about complications and side effects, questions remain about its effectiveness (37), some say that it may be considered for palliating neurological symptoms.
If local symptoms in the brain are severe, that is, if both brain metastasis and dural metastasis or epilepsy are present, WBRT with irradiation such as 3 Gy × 10 fractions or 4 Gy × 5 fractions is selected (38).
Furthermore, in cases where cranial nerve damage is the main symptom, WBRT combined with steroid therapy is also recommended. Diplopia improves relatively quickly, however, hearing loss and facial nerve paralysis improve slowly.
Among spinal cord symptoms, local irradiation around the lumbar region is said to be effective in improving ADL performance and relieving pain, particularly in cases of spinal conus syndrome and cauda equina syndrome (39).
Details of the systemic therapy and drugs currently under investigation will be omitted as they are beyond the scope of this review.
Supportive care
At present, no definitive cure has been established for MC, so the best supportive care should be to alleviate neurological symptoms according to the condition. Narcotics are often chosen to manage pain caused by spinal cord symptoms and associated bone lesions, and in many cases, the dosage is gradually increased or added according to the condition. However, MC interferes with the patient’s dignity and ADL performance due to severe neurological symptoms, thus, pain relief and psychological relief are not enough to support the patient with a neurological deficit. Epilepsy is also relatively common in MC and may range from generalized tonic convulsions to nonconvulsive epilepsy and may not be noticed. Some cases may require antiepileptic drugs (40,41). Steroids are also useful for sciatica caused by spinal cord lesions and cranial nerve disorders. Neurological symptoms must be alleviated as much as possible.
Conclusions
MC in breast cancer can develop with complaints of decreased appetite and nausea even in the absence of chemotherapy, headaches that worsen daily, and progressive impairment of consciousness of unknown causes, making it difficult for cancer doctors to diagnose the condition. Despite the introduction of certain drugs that are effective against MC, such as ADCs and TKIs, their effectiveness is still limited. With this lack of established treatment, the neurological symptoms must be alleviated. For the diagnosis and palliative treatment, the pathological condition was classified into five categories, and each treatment was reviewed in the literature, however, not many studies have reported on the neurological symptoms management. Standard methods for the neurological symptoms management, such as management of hydrocephalus, meningitis symptoms, cranial nerve signs, and spinal cord damages, should be established. Although systemic therapy will be likely established in the future, until then, methods for the neurological symptoms management must be individualized.
Acknowledgments
None.
Footnote
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-47/prf
Funding: None.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-47/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The images shown in this review are from cases at the Niigata Cancer Center Hospital. The patients were informed in advance of their consent for use in research and reporting.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol 2010;11:871-9. [Crossref] [PubMed]
- Sacco K, Muhammad A, Saleem W, et al. Leptomeningeal carcinomatosis as the primary presentation of relapse in breast cancer. Oncol Lett 2016;12:779-82. [Crossref] [PubMed]
- Lin NU, Borges V, Anders C, et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J Clin Oncol 2020;38:2610-9. [Crossref] [PubMed]
- Kuksis M, Gao Y, Tran W, et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol 2021;23:894-904. [Crossref] [PubMed]
- Takahashi H, Isogawa M. Management of breast cancer brain metastases. Chin Clin Oncol 2018;7:30. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Griguolo G, Pouderoux S, Dieci MV, et al. Clinicopathological and Treatment-Associated Prognostic Factors in Patients with Breast Cancer Leptomeningeal Metastases in Relation to Tumor Biology. Oncologist 2018;23:1289-99. [Crossref] [PubMed]
- Brook RC, Tung K, Oeppen R. Batson’s plexus and retrograde venous spread of malignancy–a pictorial review. Cancer Imaging 2014;14:40. [Crossref]
- Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972-7. [Crossref] [PubMed]
- Lai R, Dang CT, Malkin MG, et al. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer 2004;101:810-6. [Crossref] [PubMed]
- Lin NU, Murthy RK, Abramson V, et al. Tucatinib vs Placebo, Both in Combination With Trastuzumab and Capecitabine, for Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer in Patients With Brain Metastases: Updated Exploratory Analysis of the HER2CLIMB Randomized Clinical Trial. JAMA Oncol 2023;9:197-205. Erratum in: JAMA Oncol 2023;9:284. [Crossref] [PubMed]
- Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 2022;28:1840-7. [Crossref] [PubMed]
- Morris CD, Humphrey C, Dillon P. A comprehensive review of current treatment modalities for leptomeningeal carcinomatosis in breast cancer. Crit Rev Oncol Hematol 2024;204:104513. [Crossref] [PubMed]
- Terasaki K, Mizuno C, Fujiishi S, et al. Garcin Syndrome Due to Meningeal Carcinomatosis from Gastric Cancer. Intern Med 2021;60:855-8. [Crossref] [PubMed]
- Chamberlain MC, Kormanik PA, Glantz MJ. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro Oncol 2001;3:42-5. [Crossref] [PubMed]
- Koc I, Temel M, Kokoglu S, et al. Leptomeningeal carcinomatosis presenting with acute motor axonal neuropathy. Indian J Cancer 2022;59:552-5. [Crossref] [PubMed]
- Thakkar JP, Kumthekar P, Dixit KS, et al. Leptomeningeal metastasis from solid tumors. J Neurol Sci 2020;411:116706. [Crossref] [PubMed]
- Mitsuya K, Nakasu Y, Deguchi S, et al. FLAIR hyperintensity along the brainstem surface in leptomeningeal metastases: a case series and literature review. Cancer Imaging 2020;20:84. [Crossref] [PubMed]
- Kurihara M, Koda H, Aono H, et al. Rapidly progressive miliary brain metastasis of lung cancer after EGFR tyrosine kinase inhibitor discontinuation: An autopsy report. Neuropathology 2019;39:147-55. [Crossref] [PubMed]
- Lee SH, Kong DS, Seol HJ, et al. Ventriculoperitoneal shunt for hydrocephalus caused by central nervous system metastasis. J Neurooncol 2011;104:545-51. [Crossref] [PubMed]
- Jung TY, Chung WK, Oh IJ. The prognostic significance of surgically treated hydrocephalus in leptomeningeal metastases. Clin Neurol Neurosurg 2014;119:80-3. [Crossref] [PubMed]
- Hochmair M. Medical Treatment Options for Patients with Epidermal Growth Factor Receptor Mutation-Positive Non-Small Cell Lung Cancer Suffering from Brain Metastases and/or Leptomeningeal Disease. Target Oncol 2018;13:269-85. Erratum in: Target Oncol 2018;13:667. [Crossref] [PubMed]
- Liao BC, Lee JH, Lin CC, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Non-Small-Cell Lung Cancer Patients with Leptomeningeal Carcinomatosis. J Thorac Oncol 2015;10:1754-61. [Crossref] [PubMed]
- Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14:64-71. [Crossref] [PubMed]
- Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J Clin Oncol 2019;37:1081-9. [Crossref] [PubMed]
- Pellerino A, Soffietti R, Bruno F, et al. Neratinib and Capecitabine for the Treatment of Leptomeningeal Metastases from HER2-Positive Breast Cancer: A Series in the Setting of a Compassionate Program. Cancers (Basel) 2022;14:1192. [Crossref] [PubMed]
- Pérez-García JM, Vaz Batista M, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol 2023;25:157-66. [Crossref] [PubMed]
- Niikura N, Yamanaka T, Nomura H, et al. Treatment with trastuzumab deruxtecan in patients with HER2-positive breast cancer and brain metastases and/or leptomeningeal disease (ROSET-BM). NPJ Breast Cancer 2023;9:82. [Crossref] [PubMed]
- Tolaney SM, Sahebjam S, Le Rhun E, et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor-Positive Breast Cancer. Clin Cancer Res 2020;26:5310-9. [Crossref] [PubMed]
- Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 2016;34:2460-7. [Crossref] [PubMed]
- Khosla AA, Saxena S, Ozair A, et al. Novel Therapeutic Approaches in Neoplastic Meningitis. Cancers (Basel) 2022;15:119. [Crossref] [PubMed]
- Modi S, Saura C, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 2020;382:610-21. [Crossref] [PubMed]
- Boogerd W, van den Bent MJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer 2004;40:2726-33. [Crossref] [PubMed]
- Sause WT, Crowley J, Eyre HJ, et al. Whole brain irradiation and intrathecal methotrexate in the treatment of solid tumor leptomeningeal metastases--a Southwest Oncology Group study. J Neurooncol 1988;6:107-12. [Crossref] [PubMed]
- Sandberg DI, Bilsky MH, Souweidane MM, et al. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery 2000;47:49-54; discussion 54-5. [PubMed]
- Zairi F, Le Rhun E, Bertrand N, et al. Complications related to the use of an intraventricular access device for the treatment of leptomeningeal metastases from solid tumor: a single centre experience in 112 patients. J Neurooncol 2015;124:317-23. [Crossref] [PubMed]
- Bousquet G, Darrouzain F, de Bazelaire C, et al. Intrathecal Trastuzumab Halts Progression of CNS Metastases in Breast Cancer. J Clin Oncol 2016;34:e151-5. [Crossref] [PubMed]
- Graham PH, Bucci J, Browne L. Randomized comparison of whole brain radiotherapy, 20 Gy in four daily fractions versus 40 Gy in 20 twice-daily fractions, for brain metastases. Int J Radiat Oncol Biol Phys 2010;77:648-54. [Crossref] [PubMed]
- Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017;28:iv84-99. [Crossref] [PubMed]
- Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol 2012;13:e375-82. [Crossref] [PubMed]
- Assi HI, Mahmoud T, Saadeh FS, et al. Management of leptomeningeal metastasis in breast cancer. Clin Neurol Neurosurg 2018;172:151-9. [Crossref] [PubMed]
Cite this article as: Takahashi H. Neurological symptom management in breast cancer meningeal carcinomatosis. Transl Breast Cancer Res 2025;6:7.


