
Radiotherapy in breast cancer patients achieving nodal pathologic complete response after neoadjuvant therapy: a scoping review
Highlight box
Key findings
• Most of the studies evaluated in this scoping review failed to show significant benefit from the addition of radiotherapy in ypN0 cases, particularly in cases with clinical criteria similar to those of the NSABP B-51 study, i.e., initially cT1-3 cN1, in which nodal response was achieved following neoadjuvant chemotherapy (NAC).
What is known and what is new?
• Traditionally, regional nodal irradiation (RNI) or postmastectomy radiotherapy (PMRT) for patients receiving NAC was guided by the disease volume at initial diagnosis, without considering the patient’s response to the neoadjuvant systemic treatment, but rather relying on data from patients who underwent upfront surgery.
• Currently, there has been much debate on the role of radiotherapy in the presence of complete lymph node response (ypN0) to NAC. This review shows that no benefit is gained from RNI or PMRT in ypN0 cases. Conversely, in cases of initially more advanced clinical staging (cT4 cN2/3) or residual lymph node disease, radiotherapy should be recommended.
What is the implication, and what should change now?
• The possibility of de-escalating radiotherapy as a localized treatment following NAC is supported by these findings. Nevertheless, the gap in information persists, with further data from randomized studies being required.
Introduction
Background
Neoadjuvant chemotherapy (NAC), traditionally recommended for locally advanced breast cancer, has recently been used in cases of operable disease. This change in management increased the rate of breast-conserving surgery (BCS) to the point of including cases initially ineligible, with the benefit of not affecting overall survival when compared to adjuvant chemotherapy (1,2). Axillary surgery was also impacted by NAC. Even in cases of clinically positive axilla (cN+) at initial diagnosis, sentinel lymph node biopsy (SLNB) can be performed following complete clinical response (3-8). NAC also provides an opportunity to evaluate the “in vivo” response, and prognosis in terms of disease-free survival and overall survival can be estimated by evaluating pathologic complete response (pCR), with additional adjuvant therapies being used in cases of residual disease (9-11). In recent years, interest has grown in investigating the possibility of de-escalating localized treatment such as surgery and radiotherapy following NAC (12,13).
Rationale and knowledge gap
Indications for adjuvant radiotherapy at upfront surgery are based on disease volume at the time of surgery. In addition to whole breast irradiation (WBI) following BCS, postmastectomy radiotherapy (PMRT) is recommended for breast cancer patients in cases of tumors over 5 cm and with four or more positive lymph nodes (14). More recently, the benefit of PMRT was demonstrated in women with 1–3 affected lymph nodes. A meta-analysis of 22 studies found that PMRT reduced 10-year locoregional recurrence rates [odds ratio (OR): 0.68; 95% confidence interval (CI): 0.57–0.82] and 20-year mortality rates (OR: 0.80; 95% CI: 0.67–0.95) (15). Regional nodal irradiation (RNI) can also reduce breast cancer recurrence, particularly in cases of node-positive disease (16). On the other hand, although well-established at upfront surgery, the role of radiotherapy following NAC has been a subject of debate. Indeed, the initial disease volume, which constitutes the key criterion for recommending radiotherapy at upfront surgery, is altered by neoadjuvant systemic treatment as it affects the tumor and lymph nodes (17). These changes are particularly prominent following the advent of new systemic treatments that have increased pCR rates (18-20).
Traditionally, RNI or PMRT for patients receiving NAC was guided by the same treatment criterion used for cases of upfront surgery, i.e., only the initial disease volume was considered, irrespective of the patient’s response to the neoadjuvant systemic treatment (14,15). In this respect, some retrospective studies showed the benefit of radiotherapy in locoregional control, particularly in locally advanced disease prior to NAC (21).
A combined analysis of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 studies, however, presented a new scenario. Many of the patients included in those analyses (n=1,071) did not undergo PMRT, even under the current criteria for its indication. Eight-year locoregional recurrence rates differed between patients without nodal response and those with axillary pCR (14.9% and 5.9%, respectively) (22). Those results were the reasoning behind the NSABP-51 study that was recently presented at the 2023 San Antonio Breast Cancer Symposium (23). Although the results of that randomized study have yet to be published, there has been much debate on the role of radiotherapy in the presence of complete lymph node response (ypN0).
Objective
This study conducted a scoping review of contemporary studies designed to evaluate the impact of radiotherapy on patients submitted to NAC who achieved pCR in the breast and/or axilla, based on the NSABP B-51 criteria (initially cT1-3 cN1, achieving nodal response following NAC). We present this article in accordance with the PRISMA-ScR reporting checklist (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-54/rc).
Methods
Reports on the radiotherapy treatment of patients receiving NAC who achieved pCR in the breast and/or axilla, based on the NSABP B-51 criteria, were sought in the Medline/PubMed, LILACS and ClinicalTrials.gov databases between April and June 2024. The following keywords were used in accordance with research conducted in the Virtual Health Library using the Health Sciences Descriptors (DeCS) and Medical Subject Headings (MeSH): “breast neoplasms”, “neoadjuvant therapy”, “neoadjuvant chemotherapy”, “mastectomy”, “segmental mastectomy”, “radiotherapy”, “postmastectomy radiotherapy”, “regional nodal irradiation” and “drainage radiotherapy”. The search was limited to studies published in English over the preceding 14 years (January 2010 to May 2024). Additionally, a manual search of the references cited in the reviewed papers was conducted to identify more articles.
The inclusion criteria were: (I) randomized and non-randomized clinical trials and retrospective studies with over 200 patients that evaluated oncological outcomes (at least locoregional recurrence, disease-free survival and overall survival), preferably calculated over a period of at least five years; (II) the impact of PMRT and/or RNI in ypN0 cases following NAC should be described, mirroring the NSABP B-51 criteria. However, data on positive lymph nodes (ypN+) following NAC were also evaluated when available in the same study; and (III) studies published in English.
The exclusion criteria were: (I) studies that included cases of upfront surgery; (II) studies that did not report the impact of PMRT and/or RNI in ypN0 cases following NAC according to the NSABP B-51 criteria; and (III) studies published in languages other than English.
The data were consolidated using a scoping approach and a table was constructed to describe the principal characteristics of each study included in the review. After the articles had been selected and read in their entirety by at least two of the authors, those identified for inclusion were separated according to the study design (retrospective, non-randomized prospective or randomized prospective) and the type of radiotherapy (PMRT and RNI). This description was to be finalized according to the summary of the NSABP B-51 study.
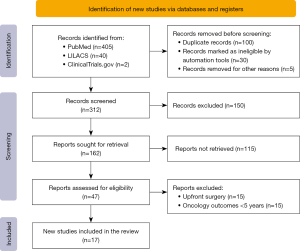
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) were followed throughout the entire review process (24,25). An initial search revealed 447 studies. Of these, 405 were found in PubMed, 40 in LILACS and 2 in ClinicalTrials.gov. After duplicated articles had been removed, the total number of studies was reduced to 312. Screening of the titles and abstracts resulted in the exclusion of a further 295 studies. The complete articles for the remaining 47 studies were obtained. After application of the inclusion and exclusion criteria, 17 studies were included in the final analysis (Figure 1).
Results
Seventeen articles were selected for inclusion, 15 retrospective cohort studies, one prospective cohort study, and the NSABP B-51, a randomized controlled trial (Table 1) (23,26-41). Most of the studies (n=10) evaluated PMRT alone, while 4 evaluated PMRT and RNI and 3 analyzed only RNI.
Table 1
| Author | Year of publication | Design | Period of treatment | Sample (n) | Type of radiotherapy | Benefit ypN0 |
|---|---|---|---|---|---|---|
| Rusthoven et al. (26) | 2016 | Retrospective cohort | 2003–2011 | 15,315 | PMRT/RNI | Yes (PMRT; OS) |
| Liu et al. (27) | 2016 | Retrospective cohort | 1998–2009 | 1,560 | PMRT | Yes (initial EC III, T3/4 OS) |
| Kantor et al. (28) | 2017 | Retrospective cohort | 2004–2008 | 8,321 | PMRT | Yes (HR-negative, OS) |
| Haffty et al. (29) | 2019 | Retrospective cohort | 2009–2011 | 701 | PMRT/RNI | No |
| Krug et al. (30) | 2019 | Retrospective cohort | 2002–2010 | 817 | PMRT | Yes (initial T3/T4; LRR) |
| Miyashita et al. (31) | 2019 | Retrospective cohort | 2004–2009 | 3226 | PMRT | No |
| Cho et al. (32) | 2019 | Retrospective cohort | 2005–2011 | 261 | RNI | No |
| Fayanju et al. (33) | 2020 | Retrospective cohort | 2004–2015 | 26,009 | RNI | No |
| Huang et al. (34) | 2020 | Retrospective cohort | 2000–2014 | 1,813 | PMRT | No |
| Zhang et al. (35) | 2021 | Retrospective cohort | 2007–2015 | 554 | PMRT | No |
| Haque et al. (36) | 2021 | Retrospective cohort | 2004–2017 | 14,690 | PMRT | No |
| Schlafstein et al. (37) | 2022 | Retrospective cohort | 2004–2015 | 1,963 | RNI | No |
| Kim et al. (38) | 2022 | Retrospective cohort | 2013–2017 | 807 | PMRT | Yes (triple-negative tumors; LRR) |
| de Wild et al. (39) | 2022 | Prospective cohort | 2011–2015 | 838 | PMRT/RNI | No |
| Saifi et al. (40) | 2023 | Retrospective cohort | 2009–2011 | 312 | PMRT | No |
| Tan et al. (41) | 2024 | Retrospective cohort | 2008–2019 | 333 | PMRT | No |
| Mamounas et al. (23) | 2024* | Randomized controlled trial | 2013–2020 | 1,641 | PMRT/RNI | No |
*, presented at San Antonio Breast Cancer Symposium. EC, clinical staging; HR, hormone receptor; LRR, locoregional recurrence; OS, overall survival; PMRT, postmastectomy radiotherapy; RNI, regional nodal irradiation.
Retrospective studies that evaluated both PMRT and RNI
One study, conducted with 15,315 cases between 2011 and 2013 using the National Cancer Database (NCDB), evaluated the impact of PMRT and RNI in patients with positive axillary nodes at initial diagnosis (cT1-3 cN1 M0) who were then treated with NAC (26). The cases were stratified into four cohorts based on the type of surgery (mastectomy or BCS) and lymph node status following NAC (ypN0 or ypN+): 3,040 cases of mastectomy/ypN0, 7,243 cases of mastectomy/ypN+, 2,070 cases of BCS/ypN0 and 2,962 cases of BCS/ypN+. Overall survival was better in the group that received PMRT irrespective of the lymph node pathologic response to NAC (ypN+; P<0.001/ypN0; P=0.01), with this benefit persisting in the multivariate analysis. Conversely, there was no difference in overall survival with the association of RNI in cases of BCS.
The role of PMRT and RNI on locoregional recurrence in patients submitted to NAC with positive axillary nodes at initial diagnosis (cT0-4 cN1-2) was evaluated in the American College of Surgeons Oncology Group (ACOSOG) Z1071 study (29). That study included patients between 2009 and 2011 who had undergone SLNB followed by axillary lymph node dissection (ALND). Of the 701 patients included in the analysis, mastectomy was performed in 423 cases (59.6%), while BCS was performed in 277 (40.4%). Overall, of the patients treated with BCS, 153 (22.1%) underwent RNI, while 118 (17.1%) did not. Of the cases of mastectomy, 347 (50.2%) received PMRT and 73 (10.6%) did not. After a mean follow-up time of 5.9 years (range, 0.4–8.1 years), locoregional recurrence was detected in 43 patients (6.1%), distant recurrence in 145 (20.7%), and 142 patients died (20.4%). Locoregional recurrence rates were generally lower in patients who received PMRT or RNI [hazard ratio (HR) =2.35; P=0.01], with no differences in overall survival or disease-free survival. There was no statistically significant difference in locoregional recurrence in those cases in which pCR was achieved.
Retrospective studies evaluating only PMRT
Investigation on the role of PMRT following NAC yielded conflicting results.
In a cohort from the NCDB [1998–2009] involving 1,560 patients with initial clinical stage II/III and positive axilla, who achieved axillary pCR, 903 (57.9%) received PMRT and 657 (42.1%) did not (27). After 56 months of follow-up, no differences were found in overall survival between the groups (P=0.12; HR: 1.571; 95% CI: 0.839–2.943). In the analysis of subgroups, PMRT was shown to have an impact on overall survival in cases of initial clinical stage IIIB/IIIC, initial T3/T4 and of residual disease following NAC (P<0.05). PMRT was concluded to have a heterogenous effect in patients with positive axillary nodes at initial diagnosis.
In another study, also conducted using the NCDB, PMRT impacted overall survival in hormone receptor-negative tumors, even in the ypN0 group (28). Patients with positive axillary nodes (nN1=6,140; 65.6%/cN2=2,181; 23.3%) treated with NAC and mastectomy between 2004 and 2008 and with a mean follow-up time of 69 months were evaluated. In the analysis of overall survival, PMRT was generally associated with benefit in the initially cN1 group (75.8% vs. 71.9%; P<0.01) and the initially cN2 group (69.2% vs. 58.6%; P<0.01). Nevertheless, when the patients with nodal response (ypN0) were evaluated separately, no difference was found in survival (P>0.11) between those who underwent PMRT and those who did not, except in cases of hormone receptor-negative tumors (HR: 0.65; P<0.01).
A retrospective study also found benefit in cases of triple-negative tumors (38). Between 2013 and 2017, 682 patients with clinical stage II and III breast cancer who had undergone NAC and mastectomy were evaluated: 596 patients (87.4%) who received PMRT and 86 (12.6%) who received no radiotherapy. After a mean follow-up time of 67 months, PMRT was found to benefit ypN+ patients in terms of locoregional recurrence-free survival, disease-free survival and overall survival (P<0.001). In a multivariate analysis, factors such as histologic grade III (P=0.002), lymphovascular invasion (P=0.045) and ypN2-3 (P=0.02) were associated with locoregional recurrence. Nevertheless, no benefit was found for patients who achieved axillary pCR (ypN0) except in cases of triple-negative tumors.
A retrospective review of three randomized studies (GeparTrio, GeparQuattro and GeparQuinto) also showed some benefit of PMRT in 817 cases of non-inflammatory breast cancer submitted to NAC (PMRT: n=676; 82.7%) between 2002 and 2010 (30). Of those cases, 44.6% consisted of T3/T4 tumors and 61% had initially positive lymph nodes (cN+). In relation to tumor subtypes, most were hormone-positive/HER-negative (52.1%), followed by HER2-positive (25.2%) and triple-negative (15.7%). After a mean follow-up time of 51.5 months, the 5-year accumulated locoregional recurrence rate was 15.2% (95% CI: 9.0–22.8%) in non-irradiated patients and 11.3% (95% CI: 8.7–14.3%) in cases submitted to PMRT, with no differences between the groups in a multivariate risk analysis (P=0.23). For initially T3/T4 patients, however, locoregional recurrence rates were lower following radiotherapy (HR: 0.43; 95% CI: 0.19–0.94; P=0.04), with borderline benefit (HR: 0.37; 95% CI: 0.14–0.99; P=0.04) for cases that achieved axillary pCR (cN+ to ypN0: n=158).
Conversely, other studies failed to find any benefit in patients who achieved axillary response. A study conducted using the Japanese Breast Cancer Registry evaluated the effectiveness of radiotherapy in cT1-4 cN0-2 M0 patients treated with NAC and mastectomy (31). The objective was to evaluate the association between radiotherapy and locoregional recurrence, distant disease-free survival and overall survival based on nodal status (ypN) following NAC in a multivariate analysis. Overall, 3,226 patients met the inclusion criteria, with 993 of these being in the PMRT group. Mean age was 53 years (range, 23–92 years), with 1,387 patients (43%) being premenopausal. A total of 1,413 patients (43.8%) had cT2 tumors, while 665 (20.6%) had cT3 tumors and 897 (27.8%) had cT4 tumors. In relation to initial lymph node status, 1,759 cases (54.5%) were cN1 and 379 (11.4%) cN2, while 828 (25.6%) were cN0. After a 5-year follow-up time, 412 patients (12.8%) had presented with locoregional events, 679 (21%) experienced distant recurrence and 561 (17.4%) had died. Five-year locoregional recurrence rates for ypN0 with and without radiotherapy were 7.0% and 6.7%, respectively, while rates for ypN1 with and without radiotherapy were 12.5% and 13.1%, respectively. The association between radiotherapy and overall survival was not statistically significant for ypN0 (P=0.22) or ypN1 patients (P=0.51). Radiotherapy was associated, on the other hand, with locoregional recurrence-free survival (P<0.001), distant recurrence-free survival (P=0.01) and overall survival (P<0.001) in ypN2-3 patients. The multivariate analysis showed that radiotherapy was associated with locoregional recurrence-free survival (HR: 0.61; 95% CI: 0.45–0.82; P=0.001) and overall survival (HR: 0.69; 95% CI: 0.53–0.89; P=0.004) in cases of ypN2-3 alone.
Another study that evaluated the role of PMRT in patients submitted to NAC with stage II and III breast cancer and affected lymph nodes also found no evidence of any significant benefit in cases of ypN0 (34). That analysis consisted of a retrospective review of 1,813 cT1-4N1-2M0 patients treated in 13 institutes and divided into three groups based on lymph node response following NAC (ypN0, ypN1 and ypN2-3). Of those patients, 490 (27.0%) were in the ypN0 group, 567 (31.3%) in the ypN1 group and 756 (41.7%) in the ypN2-3 group. Five-year locoregional recurrence, disease-free survival and overall survival were, respectively, 86.3%, 68.4% and 83.1% for the entire cohort. PMRT influenced overall survival in patients in the ypN2-3 group (74.2% vs. 55.9%; P<0.001); however, no such benefit was found for the ypN0 (93.1% vs. 95.5%; P=0.51) or the ypN1 groups (88.4% vs. 87.8%; P=0.54).
A study conducted in a single institute in China analyzed 554 patients with initial stage II–III breast cancer submitted to NAC and mastectomy between 2007 and 2015 and reported similar results (35). The effect of PMRT on locoregional recurrence and disease-free survival rates was investigated. Mean follow-up time was 65 months and mean age was 51 years (range, 22–78 years). Overall, 395 women (71.1%) had stage II and 160 (28.9%) stage III breast cancer. Initial node status was N1 in 339 cases (61.2%) and N2 in 85 cases (15.3%). Fifty-eight cases (10.5%) had locoregional recurrence, while 138 patients (24.9%) had distant metastases and 72 (13.0%) died. A total of 399 cases (72%) were submitted to PMRT, while 155 (28%) received no radiotherapy following mastectomy. Five-year locoregional recurrence and disease-free survival rates were 9.2% and 74.2%, respectively, showing the overall positive impact of PMRT in reducing locoregional recurrence (7.3% vs. 14.1%; P=0.01). In cases with residual nodal disease (ypN1 and ypN2-3), the benefit of PMRT was significant. Nevertheless, PMRT was not found to benefit patients with negative axilla following surgery (ypN0), either in terms of locoregional recurrence or disease-free survival.
A study conducted using the NCDB evaluated the role of PMRT in patients submitted to mastectomy following NAC who met the NSABP B-51 criteria (36). Those authors did not include patients receiving RNI, since the coding for that treatment is not uniform in that database. Furthermore, they included cT4 cN2/3 patients who were excluded from the NSABP B-51. A total of 14,690 patients treated between 2004 and 2017 were included in the analysis, with 10,092 patients (69%) undergoing PMRT and 4,598 (31%) not receiving radiotherapy. Overall, 7,560 patients were under 50 years of age and 6,816 (46.4%) were cT3/4. In relation to tumor subtypes, the most common was HER2 positive (n=4,828; 32.9%), followed by triple-negative (n=4,426; 30.1%) and hormone-positive/HER2-negative tumors (n=2,956; 20.1%). After a mean follow-up time of 55.6 months (range, 34.5–82.9 months), there was no difference in estimated 10-year overall survival: 78.6% (95% CI: 77–80%) for PMRT compared to 76.3% (95% CI: 73.7–78.7%) for the non-irradiated group (P=0.41). Analysis of the specific group of cases that did not meet the criteria for the NSABP B-51 study also showed no statistically significant difference in overall survival (82.6% with PMRT vs. 80% without PMRT; P=0.25).
A combined retrospective analysis of the TRYPHAENA and NeoSphere studies (phase II studies on the addition of pertuzumab to the neoadjuvant treatment of tumors with HER2 over-expression) also evaluated the role of PMRT following NAC (40). A total of 312 patients with positive axillary nodes at initial diagnosis were analyzed. Of these, 172 patients (55%) achieved axillary pCR (ypN0), while 140 (45%) still had residual disease in the lymph nodes (ypN+). For the ypN0 patients, estimated 5-year locoregional recurrence-free survival was 97% for both groups, with or without PMRT (P=0.94). Cases of ypN1 also failed to gain any significant benefit from radiotherapy: patients who received treatment (n=40) had a locoregional recurrence-free survival rate of 85% compared to 89% for those who did not receive radiotherapy (n=22) (P=0.60). On the other hand, a significant difference in locoregional recurrence-free survival was found in ypN2-3 patients who underwent radiotherapy (n=53) compared to those who did not (n=25), with 5-year locoregional recurrence-free survival rates of 92% and 75%, respectively (P=0.01). It was concluded that cases of breast cancer with HER2 overexpression in which axillary response (ypN0) was achieved following NAC have excellent locoregional control, supporting the de-escalation of PMRT.
Finally, a recently published study conducted in a single institute in China evaluated the impact of PMRT in patients with stage II and III breast cancer, who had initial axillary disease (cN+), were submitted to mastectomy following NAC and achieved axillary pCR (ypN0) (41). Between 2008 and 2019, 333 patients were eligible, with 189 (56.8%) receiving PMRT and 144 (43.2%) no irradiation. After a mean follow-up time of 71 months, the rates of locoregional recurrence-free survival, distant metastasis-free survival, breast cancer-specific survival and overall survival were, respectively, 99.1%, 93.4%, 96.4% and 94.3%. The effect of PMRT was not statistically different between those who had undergone radiotherapy and those who had not in terms of 5-year locoregional recurrence-free survival (98.9% vs. 99.2%), distant metastasis-free survival (93.8% vs. 91.3%), breast cancer-specific survival (96.7% vs. 94.9%) and overall survival (94.5% vs. 92%). There was also no difference in survival when cases were stratified according to staging (II or III).
Retrospective studies evaluating only RNI
One study used the NCDB to evaluate the role of RNI following NAC in 26,009 initially cN1 patients between 2010 and 2015. Of these, 12,341 patients achieved complete axillary response (ypN0), while 13,668 continued with residual disease in the lymph nodes (ypN1) (33). Of the ypN0 patients, 4,423 (43.9%) received RNI compared to 7,556 (55.3%) of the ypN1 patients. In cases submitted to mastectomy, RNI had no effect on overall survival in the ypN0 group (P=0.20); however, there was a statistically significant benefit for ypN1 patients (HR: 0.83; 95% CI: 0.69–0.99; P=0.04). Conversely, for those who underwent BCS, RNI was associated with a positive effect on both ypN0 patients (HR: 0.38; 95% CI: 0.22–0.66) and ypN1 patients (HR: 0.44; 95% CI: 0.30–0.66); however, there was no additional benefit to patients who also underwent WBI.
A multicenter retrospective study conducted in Korea evaluated the role of RNI in 261 patients from 13 institutes who were submitted to NAC and BCS and achieved axillary pCR (ypN0) (32). The tumor subtypes were triple-negative (n=113; 43.3%), HER2 positive (n=95; 36.4%) and luminal (n=53; 20.3%). Overall, 108 patients achieved pCR, including ypT0 and ypTis. After a mean follow-up time of 79 months (range, 16–141 months), the estimated rates of locoregional recurrence, disease-free survival and overall survival were 96.0%, 91.0% and 96.8%, respectively. RNI had no effect on locoregional recurrence, disease-free survival or overall survival, irrespective of the tumor subtype or whether pCR was achieved in the breast. The benefit of RNI was also evaluated in the patients who underwent SLNB alone compared to those submitted to ALND, with no evidence being found of any benefit (P=0.38 in the SLNB group and P=0.46 in the ALND group).
Finally, another study sought to compare oncologic outcomes in women submitted to BCS and WBI, with or without RNI, who had initially positive axilla (cN1) and achieved complete axillary response (ypN0; SLNB alone) following NAC (37). Using the NCDB for the 2006–2015 period, 1,963 patients who met the criteria for analysis were identified, with 946 of these having undergone RNI and 1,017 breast radiotherapy alone. There was an increase in the indications for RNI over that period from 48.13% in 2004 to 62.13% in 2015 (P<0.001). Nevertheless, according to that analysis, the trend was not accompanied by any clinical benefit: estimated 10-year overall survival for the group of patients who underwent RNI was 79.5% (95% CI: 72.9–84.6%) compared to 83.6% (95% CI: 78.1–87.9%) for those who did not (P=0.14).
Nonrandomized prospective study
The RAPCHEM (BOOG 2010-03) was a prospective, multicenter, nonrandomized study conducted in the Netherlands on the role of radiotherapy following NAC in patients with cT1-2 cN1 breast cancer at initial presentation between 2011 and 2015 (39). The study included 848 patients from 17 centers treated with primary chemotherapy followed by breast and axillary surgery. Axillary status was confirmed by biopsy prior to the initiation of treatment. The patients were divided into three predefined risk groups for locoregional recurrence: low (ypN0), intermediate (ypN1) or high (ypN2-3). The low-risk group underwent WBI in cases of BCS, while the intermediate risk group was treated with localized radiotherapy following BCS or chest wall radiotherapy following mastectomy, with the addition of irradiation of axillary lymph node levels I–II in cases in which ALND was omitted. The high-risk group received RNI as well as WBI in cases of BCS or chest wall radiotherapy in cases of mastectomy. Radiotherapy for the internal mammary nodes was optional. The authors speculated that the 5-year locoregional recurrence rate would be under 4%, with an internal upper 95% confidence limit below 7.8, thus requiring 237 patients in each risk group for a statistical power of 80%. The final analysis included 291 patients in the low-risk group, 370 in the intermediate-risk group and 177 in the high-risk group. Median age was 49 years. In relation to subtypes, 64% of patients had hormone receptor-positive/HER2-negative tumors, while 13% of cases were hormone receptor-positive/HER2 positive, 7% were hormone receptor-negative/HER2-positive and 15% were triple-negative. After a mean follow-up time of 5.8 years, the 5-year locoregional recurrence rate was 2.2% (95% CI: 1.4–3.4%) for the entire group, with no difference between the different risk groups: locoregional recurrence was 2.1% (0.9–4.3%) in the low-risk group, 2.2% (1.0–4.1%) in the intermediate-risk group and 2.3% (0.8–5.5%) in the high-risk group. In a post hoc analysis conducted with patients treated according to the recommended study treatment guidelines, the primary outcome of the study was also achieved as previously estimated, with locoregional recurrence rates of 2.3% (0.8–5.3%), 1.0% (0.2–3.4%) and 1.4% (0.3–4.5%) for the low, intermediate and high-risk groups, respectively. RAPCHEM was the first prospective study to report data on de-escalating radiotherapy according to patient response following NAC.
Prospective randomized study
The NSABP B-51 was a phase III, multicenter, randomized clinical trial that evaluated whether patients with breast cancer and initially clinically positive axilla (cT1-3 cN1 M0) who achieved axillary pCR (ypN0) following NAC benefited from PMRT + RNI in cases of mastectomy or WBI associated with RNI in cases of BCS (23). Axillary status was determined by fine-needle aspiration or core needle biopsy. Axillary surgery following NAC could be ALND or SLNB with or without ALND. The patients were stratified according to type of surgery, hormone receptor status, HER2 status, whether adjuvant chemotherapy was given and whether pCR of the breast was achieved. In the radiotherapy group, PMRT + RNI was given in cases of mastectomy and WBI + RNI in cases of BCS, while the experimental group was given radiotherapy alone in cases of BCS. The primary outcome was the invasive breast cancer recurrence-free interval (IBCRFI). The IBCRFI was defined as local, regional or distant invasive recurrence or breast cancer-specific death. Secondary endpoints were the isolated locoregional recurrence-free interval, distant recurrence-free interval, disease-free survival, overall survival and toxicity. Between 2013 and 2020, 1,556 patients (RNI: 772/no RNI: 784) were analyzed. Around 40% of the patients were ≤49 years of age; 20% of cases were cT3; 21% were triple-negative and 56% were HER2 positive. Mastectomy was the form of surgery performed in 42% of cases, and SLNB was the axillary procedure performed in 55%. In 22% of cases, pCR of the breast was not achieved and adjuvant chemotherapy was given in only 1% of cases. After a mean follow-up time of 59.5 months (range, 40.7–74.1 months), there was no difference between the groups with respect to the primary study outcome (IBCRFI): the estimated 5-year rate was 92.7% (50 events) in the RNI group compared to 91.8% (59 events) in the group of patients who did not receive radiotherapy (HR: 0.88; 95% CI: 0.60–1.29; P=0.51). Evaluation of the secondary endpoints showed no statistically significant difference between the groups (Table 2). Evaluation of the subgroups with respect to the principal outcome showed no differences in relation to age (P=0.09), type of breast surgery (P=0.28), type of axillary surgery (P=0.42), hormone receptor status (P=0.17), HER2 overexpression (P=0.47) or pCR of the breast (P=0.59). On the other hand, the triple-negative subtype appeared to benefit more in the non-irradiated group (HR: 2.30; 95% CI: 1.00–5.25), while with the hormone-positive subtype there was a trend towards an advantage with irradiation (HR: 0.41; 95% CI: 0.17–0.99); however, the small number of events in these analyses has to be taken into account. No deaths or unexpected intoxications related to the treatment occurred.
Table 2
| Outcome | Events (%) | HR (95% CI) | P value | |
|---|---|---|---|---|
| RNI | No RNI | |||
| IBCRFI | 50 (92.7) | 59 (91.8) | 0.88 (0.60–1.29) | 0.51 |
| ILRRFI | 4 (99.3) | 11 (98.4) | 0.37 (0.12–1.16) | 0.088 |
| DRFI | 46 (93.4) | 48 (93.4) | 1.00 (0.67–1.51) | 0.99 |
| DFS | 85 (88.3) | 83 (88.5) | 1.06 (0.79–1.44) | 0.69 |
| OS | 49 (93.6) | 45 (94.0) | 1.12 (0.75–1.68) | 0.59 |
RNI: regional radiotherapy (PMRT/nodal with mastectomy and nodal with breast-conserving surgery); no RNI (only radiotherapy of the breast at breast-conserving surgery). CI, confidence interval; DFS, disease-free survival; DRFI, distant recurrence-free interval; HR, hazard ratio; IBCRFI, invasive breast cancer recurrence-free interval; ILRRFI, isolated locoregional recurrence-free interval; NSABP, National Surgical Adjuvant Breast and Bowel Project; OS, overall survival; RNI, regional nodal irradiation.
Discussion
Key findings
This scoping review further supports the de-escalation of PMRT and RNI in selected patients who achieve axillary pCR following NAC. The prospective studies evaluated here, as well as most of the retrospective studies, with some exceptions (26-28,30,38), failed to show any significant benefit from the addition of radiotherapy in ypN0 cases, particularly in cases with clinical criteria similar to those of the NSABP B-51 study, the first randomized clinical trial presented in this scenario; however, yet to be published in its entirety (23).
Strengths and limitations
There are some limitations associated with this review that need to be mentioned. First, only one randomized study was included (NSABP B-51), the results of which have yet to be published in full, with only the abstract and the paper presented at the San Antonio Symposium being presently available. Most of the other studies included are retrospective longitudinal studies with considerable heterogeneity, hence hampering the evaluation of many factors, including cases of tumor stage T3, many of which were analyzed in conjunction with T4. The description of the mastectomy technique used, as well as the type of axillary surgery, constitutes another limitation. At the beginning of the NSABP B-51 study, axillary dissection was the standard, even after clinical response and axillary pCR. There are no data on the technique used in SLNB and it is unknown whether the false-negative rate could have affected the outcomes in this respect.
The strengths of this review include the fact that the studies examined here involved patients who underwent modern systemic therapy between January 2010 and May 2024, having, therefore, a greater chance of achieving pCR after NAC. No studies involving patients who underwent upfront surgery were included. Two prospective studies were included, one of which was a randomized phase III trial. To the best of our knowledge, this is the first review of its kind to incorporate findings from the NSABP B-51 trial into this analysis.
Comparison with similar research
These data show the impact of systemic therapy on the prognosis of these patients due to the reduction in disease volume after NAC. This contradicts previous understanding that radiotherapy should be recommended based on the disease volume at the time of diagnosis. Although most studies included in this review are retrospective, real-world data are welcome and valid for governmental organizations and medical societies (42-44). Nevertheless, gaps in information persist, leading the way for discussions on the benefit of radiotherapy.
Explanations of findings
One of the principal points of debate on the results of the NSABP B-51 study focuses on the absence of any benefit of radiotherapy on initially T3 tumors, traditionally associated with a significant effect at upfront surgery (23). Indeed, several of the retrospective studies evaluated in this analysis have found a benefit in initially more advanced tumors, even in ypN0 following NAC (27,30). On the other hand, retrospective studies prevent a more reliable evaluation in this setting due to their very design, with the possibility of numerous associated biases such as the joint inclusion and analysis of T3/4 tumors or even of more advanced axillary disease (cN2). Analysis of the NSABP B-51 shows that around 20% of patients in each group were T3, with no subgroup analysis of these cases being presented separately and no information on how many of these patients underwent mastectomy, although we do not believe there would be any change in this specific scenario. The NSABP B-51 did not include initially cN2 patients. A study conducted by the Italian Association of Radiation Therapy and Clinical Oncology (AIRO) evaluated the effect of loco-regional radiation therapy for operated patients in clinical stage cN2 at diagnosis and ypN0 after NAC: in this meta-analysis, the small number of studies and heterogeneity did not allow do draw definitive conclusions (45).
Another important topic of debate is the impact of radiotherapy on breast cancer with aggressive biology, particularly triple-negative tumors, even in cases of ypN0. Some analyses, including some studies contained in this review, show the particular benefit of PMRT in hormone receptor-negative breast cancer (28,38). Other studies, including the NSABP B-51, in which 20% of cases were of this subtype, failed to show any benefit of radiotherapy (PMRT or RNI) on hormone receptor-negative breast cancer following NAC and ypN0. Indeed, in the analysis of subgroups in the randomized study, these cases appeared to be those that least benefitted from radiotherapy (23). There are two points that need to be mentioned: first, the 5-year follow-up in the NSABP B-51 study appears to be sufficient to capture most of the events in this type of disease and, second, with the advent of new treatments such as neoadjuvant immunotherapy in which the rate of pCR (breast and axilla) exceeds 60%, a possible benefit of radiotherapy appears highly improbable (18). In relation to the time of follow-up, the hormone-positive subtype may demand further attention, since a considerable proportion of recurrences appear after more than five years of follow-up (46). A subject linked to these discussions is the absence of breast pCR in which some analyses may suggest some impact of radiotherapy in cases of important residual disease (ypT2 or higher). In the analysis of subgroups in the NSABP B-51, no benefit was found in 20% of the cases that failed to achieve pCR of the breast; however, the disease volume was not described separately. On the other hand, there are some drugs that could be used in adjuvant therapy that were not yet readily available for the patients with high-risk residual disease in those analyses, including capecitabine, T-DM1, abemaciclib and olaparib in cases with the BRCA mutation (10,11,47,48).
The type of surgery performed has also been a subject of debate following presentation of the NSABP B-51 study, as the absence of PMRT could affect outcomes in cases of nipple- and areola-sparing mastectomy due to the increased risk of leaving residual breast tissue in patients. In our opinion, in addition to the question that residual breast tissue following mastectomy does not necessarily imply a greater risk of recurrence, this subject is not specifically related to the benefit of PMRT but, rather, to the quality of the technique used, and cases should be evaluated on an individual basis regarding the need for this treatment (49). Indeed, up to the present time, the NSABP B-51 study has not presented information on the type of mastectomy performed, although mastectomy was carried out in 42% of cases. In relation to the axilla, in many older studies ALND was the surgical procedure used in most cases with positive axillary nodes at initial diagnosis, even when axillary pCR was achieved. More recently, following presentation of the results of some phase II studies and institutional analyses, SLNB has been performed, with very low rates of axillary recurrence irrespective of the type of tracer used (6,50,51). In the NSABP B-51, 58% of the patients underwent SLNB; however, subgroup analysis showed radiotherapy to have had no effect. Nevertheless, up to the present time, no data have been presented on the type of technique used in these cases. Conversely, the present review consistently found benefit in the studies, particularly of PMRT in cases with residual disease of the lymph nodes (ypN+), mainly in ypN2 disease. This type of disease is known to be associated with a high rate of locoregional recurrence irrespective of the type of surgery performed and it appears reasonable to affirm that radiotherapy should be routinely recommended in such cases.
Implications and actions needed
The current findings challenge the earlier belief that radiotherapy recommendations for patients undergoing NAC should be based on the disease volume at breast cancer diagnosis. However, as gaps in information remain, other studies are required on the advantages of radiotherapy.
Conclusions
The studies evaluated in conjunction in this analysis appear to show that in cases of axillary pCR (ypN0) following NAC in patients with breast cancer and initially positive axilla (cT1-3 cN1 M0), no benefit is gained from PMRT or RNI. Conversely, in cases of initially more advanced clinical staging (cT4 cN2/3) or residual lymph node disease, radiotherapy should be recommended. Further studies are required to confirm these findings.
Acknowledgments
We would like to express our sincere gratitude to the researchers and institutions responsible for the studies included in our review. Their invaluable contributions to the field of oncology and breast cancer research have provided the foundation for this work. Without their efforts in generating high-quality data and insights, this scoping review would not have been possible.
Footnote
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-54/rc
Peer Review File: Available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-54/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-24-54/coif). F.P.C. has received consulting fees from AstraZeneca, honoraria for lectures from Roche, Pfizer, Libbs, MSD, AstraZeneca, and Daiichi Sankyo, as well as support for attending meetings from MSD. Additionally, FPC is a member of the advisory boards for AstraZeneca, MSD, Pfizer, and Roche. M.A. has received honoraria for lectures from Gilead Sciences, AstraZeneca, and Daiichi Sankyo. F.P.Z. has received honoraria for lectures from AstraZeneca, Merck, Novartis, Daiichi Sankyo, and Roche, as well as support for attending meetings from AstraZeneca. Additionally, FPZ is a member of the advisory board for Merck. E.C.M. has received honoraria for lectures from Eli Lilly. A.C. has received consulting fees from Roche, Merck, Novartis, and Eli Lilly; honoraria for lectures from Roche, Merck, Daiichi Sankyo, Exact Sciences, and Eli Lilly; support for attending meetings from Merck and Roche; and stock or stock options from Eli Lilly and Novo Nordisk. In addition, AC is a member of the advisory board for Roche. F.P.B. has received honoraria for lectures from MSD, Lilly, Roche, Novo Nordisk, GC Aesthetics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015;33:258-64. [Crossref] [PubMed]
- Barrio AV, Montagna G, Mamtani A, et al. Nodal Recurrence in Patients With Node-Positive Breast Cancer Treated With Sentinel Node Biopsy Alone After Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol 2021;7:1851-5. [Crossref] [PubMed]
- Cavalcante FP, Millen EC, Novita GG, et al. Sentinel lymph node biopsy following neoadjuvant chemotherapy: an evidence-based review and recommendations for current practice. Chin Clin Oncol 2023;12:6. [Crossref] [PubMed]
- Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol 2021;47:804-12. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Kuerer HM, Smith BD, Krishnamurthy S, et al. Eliminating breast surgery for invasive breast cancer in exceptional responders to neoadjuvant systemic therapy: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2022;23:1517-24. [Crossref] [PubMed]
- Loibl S, Poortmans P, Morrow M, et al. Breast cancer. Lancet 2021;397:1750-69. [Crossref] [PubMed]
- Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol 2016;34:4431-42. [Crossref] [PubMed]
- EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127-35. [Crossref] [PubMed]
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N Engl J Med 2015;373:307-16. [Crossref] [PubMed]
- Barron AU, Hoskin TL, Day CN, et al. Association of Low Nodal Positivity Rate Among Patients With ERBB2-Positive or Triple-Negative Breast Cancer and Breast Pathologic Complete Response to Neoadjuvant Chemotherapy. JAMA Surg 2018;153:1120-6. [Crossref] [PubMed]
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020;382:810-21. [Crossref] [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-84. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. [Crossref] [PubMed]
- Buchholz TA, Tucker SL, Masullo L, et al. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol 2002;20:17-23. [Crossref] [PubMed]
- Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012;30:3960-6. [Crossref] [PubMed]
- Mamounas E, Bandos H, White J, et al. Loco-regional irradiation in patients with biopsy-proven axillary node involvement at presentation who become pathologically node-negative after neoadjuvant chemotherapy: primary outcomes of NRG Oncology/NSABP B-51/RTOG 1304 [abstract]. 2023 SABCS Abstract Report. 2023;1702-1703. Available online: https://atgproductions.net/atgclients/sabcs/2023_SABCS_Abstract_Report-12-3-23.pdf
- Haddaway NR, Page MJ, Pritchard CC, et al. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev 2022;18:e1230. [Crossref] [PubMed]
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018;169:467-73. [Crossref] [PubMed]
- Rusthoven CG, Rabinovitch RA, Jones BL, et al. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: a National Cancer Database (NCDB) analysis. Ann Oncol 2016;27:818-27. [Crossref] [PubMed]
- Liu J, Mao K, Jiang S, et al. The role of postmastectomy radiotherapy in clinically node-positive, stage II-III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget 2016;7:24848-59. [Crossref] [PubMed]
- Kantor O, Pesce C, Singh P, et al. Post-mastectomy radiation therapy and overall survival after neoadjuvant chemotherapy. J Surg Oncol 2017;115:668-76. [Crossref] [PubMed]
- Haffty BG, McCall LM, Ballman KV, et al. Impact of Radiation on Locoregional Control in Women with Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy and Axillary Lymph Node Dissection: Results from ACOSOG Z1071 Clinical Trial. Int J Radiat Oncol Biol Phys 2019;105:174-82. [Crossref] [PubMed]
- Krug D, Lederer B, Seither F, et al. Post-Mastectomy Radiotherapy After Neoadjuvant Chemotherapy in Breast Cancer: A Pooled Retrospective Analysis of Three Prospective Randomized Trials. Ann Surg Oncol 2019;26:3892-901. [Crossref] [PubMed]
- Miyashita M, Niikura N, Kumamaru H, et al. Role of Postmastectomy Radiotherapy After Neoadjuvant Chemotherapy in Breast Cancer Patients: A Study from the Japanese Breast Cancer Registry. Ann Surg Oncol 2019;26:2475-85. [Crossref] [PubMed]
- Cho WK, Park W, Choi DH, et al. Role of Elective Nodal Irradiation in Patients With ypN0 After Neoadjuvant Chemotherapy Followed by Breast-Conserving Surgery (KROG 16-16). Clin Breast Cancer 2019;19:78-86. [Crossref] [PubMed]
- Fayanju OM, Ren Y, Suneja G, et al. Nodal Response to Neoadjuvant Chemotherapy Predicts Receipt of Radiation Therapy After Breast Cancer Diagnosis. Int J Radiat Oncol Biol Phys 2020;106:377-89. [Crossref] [PubMed]
- Huang Z, Zhu L, Huang XB, et al. Postmastectomy Radiation Therapy Based on Pathologic Nodal Status in Clinical Node-Positive Stage II to III Breast Cancer Treated with Neoadjuvant Chemotherapy. Int J Radiat Oncol Biol Phys 2020;108:1030-9. [Crossref] [PubMed]
- Zhang Y, Zhang Y, Liu Z, et al. Impact of Postmastectomy Radiotherapy on Locoregional Control and Disease-Free Survival in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy. J Oncol 2021;2021:6632635. [Crossref] [PubMed]
- Haque W, Singh A, Verma V, et al. Postmastectomy radiation therapy following pathologic complete nodal response to neoadjuvant chemotherapy: A prelude to NSABP B-51? Radiother Oncol 2021;162:52-9. [Crossref] [PubMed]
- Schlafstein A, Liu Y, Goyal S, et al. Regional Nodal Irradiation for Clinically Node-Positive Breast Cancer Patients With Pathologic Negative Nodes After Neoadjuvant Chemotherapy. Clin Breast Cancer 2022;22:127-35. [Crossref] [PubMed]
- Kim D, Kim JH, Kim IA, et al. Impact of Postmastectomy Radiation Therapy on Breast Cancer Patients According to Pathologic Nodal Status after Modern Neoadjuvant Chemotherapy. Cancer Res Treat 2023;55:592-602. [Crossref] [PubMed]
- de Wild SR, de Munck L, Simons JM, et al. De-escalation of radiotherapy after primary chemotherapy in cT1-2N1 breast cancer (RAPCHEM; BOOG 2010-03): 5-year follow-up results of a Dutch, prospective, registry study. Lancet Oncol 2022;23:1201-10. [Crossref] [PubMed]
- Saifi O, Bachir B, Panoff J, et al. Post-mastectomy radiation therapy in HER-2 positive breast cancer after primary systemic therapy: Pooled analysis of TRYPHAENA and NeoSphere trials. Radiother Oncol 2023;184:109668. [Crossref] [PubMed]
- Tan CF, Wang J, Zhong XR, et al. Is postmastectomy radiotherapy necessary for breast cancer patients with clinically node-positive downstaging to ypN0 after neoadjuvant chemotherapy? Breast Cancer Res Treat 2024;206:45-56. [Crossref] [PubMed]
- Lakdawalla DN, Shafrin J, Hou N, et al. Predicting Real-World Effectiveness of Cancer Therapies Using Overall Survival and Progression-Free Survival from Clinical Trials: Empirical Evidence for the ASCO Value Framework. Value Health 2017;20:866-75. [Crossref] [PubMed]
- Justo N, Espinoza MA, Ratto B, et al. Real-World Evidence in Healthcare Decision Making: Global Trends and Case Studies From Latin America. Value Health 2019;22:739-49. [Crossref] [PubMed]
- Antonini M, Mattar A, Bauk Richter FG, et al. Real-world evidence of neoadjuvant chemotherapy for breast cancer treatment in a Brazilian multicenter cohort: Correlation of pathological complete response with overall survival. Breast 2023;72:103577. [Crossref] [PubMed]
- Marino L, Lancellotta V, Franco P, et al. Loco-regional adjuvant radiation therapy in breast cancer patients with positive axillary lymph-nodes at diagnosis (CN2) undergoing preoperative chemotherapy and with complete pathological lymph-nodes response. Development of GRADE (Grades of recommendation, assessment, Development and Evaluation) recommendation by the Italian Association of radiation therapy and Clinical Oncology (AIRO). Breast 2021;55:119-27. [Crossref] [PubMed]
- Pan H, Gray R, Braybrooke J, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 2017;377:1836-46. [Crossref] [PubMed]
- Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol 2020;38:3987-98. [Crossref] [PubMed]
- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021;384:2394-405. [Crossref] [PubMed]
- Torresan RZ, dos Santos CC, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12:1037-44. [Crossref] [PubMed]
- Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J Clin Oncol 2016;34:1072-8. [Crossref] [PubMed]
- Cavalcante FP, Zerwes FP, Souza ABA, et al. The use of blue dye alone for sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initially node-positive breast cancer. Eur J Surg Oncol 2024;50:107967. [Crossref] [PubMed]
Cite this article as: Cavalcante FP, Cardoso A, Antonini M, Zerwes FP, Millen EC, Mattar A, Brenelli FP, Frasson AL. Radiotherapy in breast cancer patients achieving nodal pathologic complete response after neoadjuvant therapy: a scoping review. Transl Breast Cancer Res 2025;6:18.


