
Can Hounsfield units on chest CT characterize breast nodules as cystic or solid?
Introduction
Breast cancer is the leading cause of cancer in women in the United States, with more than 287,850 new cases diagnosed in 2022 (1). The number of chest computed tomography (CT) scans performed per year continues to increase (2) and with that increase, there is an opportunity to diagnose incidental breast cancers earlier. We recommend including the breast tissue that has been scanned on the images provided to the radiologist. In addition, we recommend that patients wear their bras during chest CT scans because the bra maintains the breasts in a reproducible position that allows comparison of breast tissue from one scan to another and also lowers the radiation to the breast (3).
Our previous research identified breast masses on chest CT in 44 of 453 women (9.7%) (4). Benign breast masses were more likely to be small in size and have low Hounsfield units (HUs) and smooth borders; cancer was more likely to be large in size, contain high HU, and less likely to have a smooth border (4). The measurement of HU on CT was helpful for identifying cystic lesions. Previous researchers have confirmed the use of HU for the diagnosis of cysts in the liver (5), kidney (6), thymus (7), and others (8). We investigate the potential of chest CT to correctly differentiate cystic from solid breast lesions.
Materials and methods
Patient selection
MModal Catalyst identified 27 women who had an ultrasound of the breast that was recommended because of a chest CT finding between January 1, 2010, and December 31, 2017.
Image analysis
All images were reviewed by a radiologist fellowship trained in both breast imaging and cardiothoracic radiology (MS). Ultrasound characterization of lesion density as cystic or solid was considered the gold standard for this study. Analysis of CT scans was performed to identify lesions of interest corresponding to ultrasound abnormality; average, minimum, and maximum HUs were measured using the VUEPACs software program (Philips, Amsterdam, the Netherlands). Patient demographic data were obtained from the chart, including age and lesion type.
Statistical analysis
Statistical analysis was performed using Microsoft Excel v.16.48 with an advanced data analysis package (Redmond, WA, USA). Student t-tests were used to evaluate differences between HUs of groups. For all analyses, a two-tailed P value less than or equal to 0.05 was considered significant. Analysis of variance (ANOVA) was performed to assess differences in HUs among different types of solid lesions. Multivariable regression analysis was performed to assess for variables associated with cystic versus solid lesions as determined on breast ultrasound.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Columbia University Irving Medical Center (#AAAS3915) and individual consent for this retrospective analysis was waived.
Results
CT reliably differentiates cystic from solid lesions.
We first divided the 27 lesions into cystic or solid (lesions were considered solid if they had any solid component). Our analysis included multiple types of pathologies, including cysts (Figure 1), lymphoma (Figure 2), fibroadenoma (Figure 3), and breast cancer (Figure 4). Table 1 demonstrates the average, maximum, and minimum HU on CT of solid US lesion types. Exams performed with contrast were separated from exams performed without contrast. Table 2 demonstrates the average, maximum, and minimum HU on CT of cystic US lesions. Exams performed with contrast were separated from exams performed without contrast.
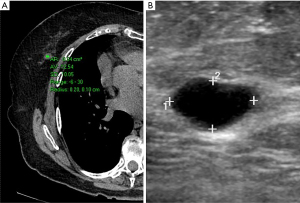
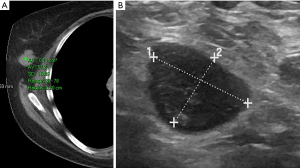
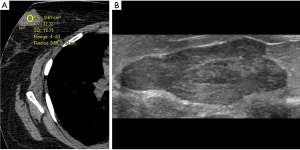
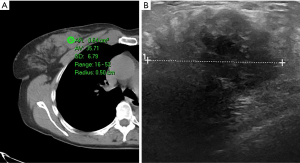
Table 1
| Nodule type | Non-contrast chest CT | Contrast chest CT | |||||
|---|---|---|---|---|---|---|---|
| Average HU | Max HU | Min HU | Average HU | Max HU | Min HU | ||
| Breast cancer | 31 | 94 | −35 | 38 | 75 | −2 | |
| 36 | 52 | 16 | 36 | 41 | 27 | ||
| 28 | 59 | 28 | 46 | 206 | −53 | ||
| 38 | 134 | −8 | – | – | – | ||
| 28 | 41 | 17 | – | – | – | ||
| Lymphoma | 36 | 89 | −6 | – | – | – | |
| 52 | 78 | 28 | – | – | – | ||
| Lymph node | – | – | – | 51 | 88 | 12 | |
| Fibroadenoma | 32 | 62 | 4 | 89 | 159 | 36 | |
| 43 | 61 | 21 | 62 | 87 | 31 | ||
| – | – | – | 37 | 47 | 29 | ||
| – | – | – | 64 | 86 | 14 | ||
| Hamartoma | 52 | 106 | 7 | 60 | 78 | 39 | |
| Fibrosis | – | – | – | 45 | 67 | 7 | |
| Average | 38 | 78 | 7 | 53 | 93 | 14 | |
CT, computed tomography; HU, Hounsfield unit; Max, maximum; Min, minimum.
Table 2
| Nodule type | Non-contrast | Contrast | |||||
|---|---|---|---|---|---|---|---|
| Average HU | Max HU | Min HU | Average HU | Max HU | Min HU | ||
| Cyst | 13 | 30 | −6 | – | – | – | |
| 27 | 38 | 18 | – | – | – | ||
| Oil cyst | – | – | – | 37 | 37 | 24 | |
| Abscess | – | – | – | 11 | 29 | −4 | |
| Seroma | 16 | 46 | −27 | 12 | 29 | −10 | |
| – | – | – | 5 | 22 | −10 | ||
| Average | 19 | 38 | −15 | 16 | 29 | 0 | |
HU, Hounsfield unit; Max, maximum; Min, minimum.
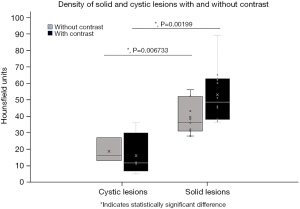
A statistically significant difference in HUs was found between the groups of cystic and solid lesions without, P=0.007, and with contrast, P=0.002 indicating that they can be reliably distinguished on CT alone (Figure 5). We examined the range of HUs for cystic and solid lesions and found a statistically significant difference between the maximum HUs (P=0.005); difference between the minimum HUs was nonsignificant (P=0.12).
Density by HUs does not differentiate types of solid lesions
We initially sought to characterize whether different types of lesions within the groups broadly characterized as cystic or solid could be differentiated by average HUs. Due to the limited number of cystic lesions within our study cohort, we focused only on solid lesions for this analysis. ANOVA evaluating each of the types of lesions did not demonstrate significant differences among the types of lesions (P=0.35).
Lesion type associated with density as measured by HUs
Finally, we sought to determine whether other factors impacted whether or not a lesion was determined to be cystic or solid. We performed multivariable regression analysis using lesion type, patient age, contrast administration, and lesion size to determine if any of these significantly contributed to lesion characterization. As expected, multivariable regression analysis demonstrated that HUs on CT were significantly associated with lesion density as measured by the gold standard ultrasound. There was no significant association between cystic versus solid density on ultrasound and the other variables listed, including patient age (P=0.93), contrast administration (P=0.73), or lesion size (P=0.85).
Discussion
The results of our study demonstrate that cystic breast lesions can be diagnosed on chest CT using HU measurements. Definitively identifying a lesion as cystic versus solid limits callbacks which are associated with patient anxiety, medical and nonmedical costs, and may result in loss to follow-up (9,10).
Breast cancer on average measured 32 HU (range, 28–36 HU). The average value increased with contrast to 40 HU (range, 38–46 HU). The average maximum HU for breast cancer was 76 HU (range, 41–134 HU) without contrast. As expected, the average maximum HU for breast cancer with contrast was higher at 107 HU (range, 41–206 HU). The minimum HU did not add value for differentiating cystic from solid lesions.
On the non-contrast studies, the highest average HUs were associated with hamartoma (53 HU), lymphoma (47 HU), followed by fibroadenoma (37 HU), and breast cancer (32 HU). Hamartoma had the highest maximum HU (106 HU) on the non-contrast study. On contrast studies, the highest average HUs were fibroadenoma (63 HU), and hamartoma (60 HU), followed by fibrosis (45 HU), and breast cancer (40 HU). Breast cancer had the highest maximum HU (107 HU) on contrast study. CT cannot reliably differentiate types of solid nodules.
On non-contrast studies, the average HU for cystic lesions was 19 compared to 38 HU for solid. The maximum HU for cystic was 38 HU and solid 78 HU. Cystic lesions did not change with contrast (average HU 16, maximum HU 29). Solid lesions enhanced with contrast and the gap between cystic and solid lesions was widened (average HU 53, maximum HU 93) (Table 3).
Table 3
| Variables | Non-contrast | Contrast | |||
|---|---|---|---|---|---|
| Cystic | Solid | Cystic | Solid | ||
| Average HU | 19 | 38 | 16 | 53 | |
| Max HU | 38 | 78 | 29 | 93 | |
| Min HU | −5 | 7 | 0 | 14 | |
HU, Hounsfield unit; Max, maximum; Min, minimum.
The limitations of this study are that it was performed by a single reader at a single center. Evaluation yielded a subset of lesions that were cystic; a more robust analysis would have used a larger sample size, therefore, examining more cystic lesions. Statistical analysis demonstrated a robust difference between the groups and therefore a greater sample size was not necessary. Future prospective studies will determine if HU measurements of breast lesions on chest CT can avoid additional breast ultrasound examination.
Chest CT scans are performed with increased frequency and the breast parenchyma is included on these images providing the opportunity for early diagnosis of clinically occult breast cancer. HUs on chest CT can accurately differentiate cystic from solid breast lesions. They can do so on both contrast and non-contrast CT exams. Further research is needed to differentiate benign from malignant solid masses of the breast on chest CT.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.org/article/view/10.21037/tbcr-23-34/coif). M.S. has received grant funding paid to the hospital, and money for lectures and research paid to her directly. K.C. is an Advisor for Cardinal Health Oncology Summits. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Columbia University Irving Medical Center (#AAAS3915) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin 2022;72:524-41. [Crossref] [PubMed]
- Smith-Bindman R, Kwan ML, Marlow EC, et al. Trends in Use of Medical Imaging in US Health Care Systems and in Ontario, Canada, 2000-2016. JAMA 2019;322:843-56. [Crossref] [PubMed]
- Salvatore M, Margolies L, Bertolini A, et al. The need to be all inclusive: Chest CT scans should include imaged breast parenchyma. Clin Imaging 2018;50:243-5. [Crossref] [PubMed]
- Desperito E, Schwartz L, Capaccione KM, et al. Chest CT for Breast Cancer Diagnosis. Life (Basel) 2022;12:1699. [Crossref] [PubMed]
- Hwang SH, Yu JS, Chung JJ, et al. Diagnosing small hepatic cysts on multidetector CT: an additional merit of thinner coronal reformations. Korean J Radiol 2011;12:341-50. [Crossref] [PubMed]
- Agochukwu N, Huber S, Spektor M, et al. Differentiating Renal Neoplasms From Simple Cysts on Contrast-Enhanced CT on the Basis of Attenuation and Homogeneity. AJR Am J Roentgenol 2017;208:801-4. [Crossref] [PubMed]
- Ackman JB, Verzosa S, Kovach AE, et al. High rate of unnecessary thymectomy and its cause. Can computed tomography distinguish thymoma, lymphoma, thymic hyperplasia, and thymic cysts? Eur J Radiol 2015;84:524-33. [Crossref] [PubMed]
- Mittal MK, Malik A, Sureka B, et al. Cystic masses of neck: A pictorial review. Indian J Radiol Imaging 2012;22:334-43. [Crossref] [PubMed]
- Schou Bredal I, Kåresen R, Skaane P, et al. Recall mammography and psychological distress. Eur J Cancer 2013;49:805-11. [Crossref] [PubMed]
- Lowry KP, Bell S, Fendrick AM, et al. Out-of-Pocket Costs of Diagnostic Breast Imaging Services After Screening Mammography Among Commercially Insured Women From 2010 to 2017. JAMA Netw Open 2021;4:e2121347. [Crossref] [PubMed]
Cite this article as: Capaccione K, Desperito E, Asiimwe AC, Salvatore M. Can Hounsfield units on chest CT characterize breast nodules as cystic or solid? Transl Breast Cancer Res 2024;5:6.


